Fig. 1
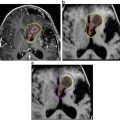
Ommaya catheter insertion demonstrated in an infant with the access point protruding from the scalp (a). T2-axial MRI brain scan of an unrelated craniopharyngioma patient demonstrating catheter tip placement in the cystic component (red arrow) (b)
A combined multicentre review of such surgical approaches from 1990 to 2000 in France (Paris, Marseille) and Brazil (Belo Horizonte, Goiânia, São Paulo) was undertaken for 50 cystic craniopharyngioma patients [17]. The cohort was predominantly paediatric with complications reported for three distinct patient groups, categorised by surgical technique. The first group (n = 26) underwent catheter placement via a frontal or pterional craniotomy under direct vision. Catheter placement was stereotactic for group two (n = 14), while group three (n = 11) had free hand catheter placement through a preceding intracranial burr hole. All catheters were connected to either a Rickham (n = 23) or an Ommaya reservoir subcutaneously. Post-operative permeability imaging (the injection of radio-opaque contrast into the cyst via a catheter) revealed either catheter misplacement or contrast leakage in eight children (16%). However, this was not significantly associated with a particular group/technique and no additional catheter placement complications were identified.
More recently, neuroendoscopic positioning of intracystic catheters has been advocated as a safer approach that open or stereotactic techniques. An Italian case series of eight patients (five paediatrics) with cystic craniopharyngioma employed this method using a Storz rigid ventriculoscope [18]. No complications were reported except for a technical failure in one child (12%) secondary to a heavily calcified cyst wall. As a consequence, the authors advocate performing a pre-operative computerised tomography (CT) head scan as opposed to the preferred MRI scan as a means of detecting such calcification. Neuroendoscopic positioning also has the advantage of enabling ventricular septostomy in cases of associated hydrocephalus to enable improved cerebrospinal fluid circulation.
The location of a craniopharyngioma and proximity to eloquent structures such as the visual apparatus and hypothalamo-pituitary axis may limit the stereotactic accessibility of a lesional cyst [21], however, combining a stereotactic endoscopic approach for intracystic catheter placement continues to be supported as a safe and effective technique, with two studies reporting operative morbidity rates of less than three percent and no peri- or post-operative mortality [19, 20].
Ultimately, the approach undertaken for intracystic catheter placement, and the subsequent outcome for the patient, is typically influenced by the expertise of the operating surgeon with a particular technique [22].
Historical Intracystic Agents Used in Cystic Craniopharyngioma
The objective behind the instillation of intracystic therapy in craniopharyngiomas is both to reduce tumour burden for the patient through cyst shrinkage and sclerose the secretive elements of the epithelial-lined cyst walls in order to prevent re-accumulation. The substances used should demonstrate efficacy at low toxicity. Prior to the introduction of interferon-alpha, agents historically used for adamantinomatous craniopharyngiomas therapy included several beta-emitting radioisotopes and the chemotherapeutic agent bleomycin (Table 1).
Table 1
Agents other than interferon-alpha used for intracystic administration in adamantinomatous craniopharyngioma—English language series involving paediatric patients
Agent | Author (year) | Number (paeds) | Median/Mean age (range–years) | CR | PR | Median/Mean F/U (range–years) | Median/Mean PFS (range–years) | Complications or toxicity |
|---|---|---|---|---|---|---|---|---|
Radioisotope P32 | Pollock (1995) [31] | 30 (10) | 35 (3–70) | 0.1 | 0.83 | 3.1 (0.6–9.7) | NR | 3—new behavioural and visual problem 3—new onset diabetes insipidus |
Hasegawa (2004) [26] | 49 (15) | 29 (3–74) | 0.17 | 0.59 | 4.1 | NR | 5—worsened pituitary deficiency 3—visual deterioration | |
Barriger (2011) [35] | 19 (NR) | 20 (3–54) | 0.05 | 0.26 | 5.2 (0.7–11.3) | 0.8 (0.1–4.5) | 6—worsened pituitary deficiency 1—new visual field deficit | |
Kickingereder (2012) [27] | 53 (19) | 31.1 (7.6–75.5) | 0.1 | 0.5 | 4.4 (0.3–16.3) | 0.8 (0.7–0.9) | 10—endocrinological progression 8—neurological deterioration 5—visual field cut deterioration 4—visual acuity deterioration 1—septic meningitis 1—cranial nerve palsy 1—hemiparesis | |
Yu (2015) [28] | 20—all paeds | Xx (0.2–14) | 0.6 | 0.4 | 4 (0.6–11.5) | 2.8 (0.5–7) | 8—other cranial nerve injury 2—optic nerve injury | |
Ansari (2015) [33] | 9—all paeds | 6.7 (3–15) | 0.23 | 0.67 | 6.7 (1–12) | 1.85 | 1—hyponatraemia | |
Radioisotope Y90 | Voges (1997) [25] | 62 (32) | 17 (4–71) | 0.45 | 0.35 | 11.9 (1.5–16.4) | NR | 3—amaurosis fugax 3—new endocrinological dysfunction 1— new visual field cut 1—death |
Julowb (2013) [29] | 78 (26) | 28 (2.9–73) | 0.2 | 0.6 | NR | NR | 59—worsened pituitary deficiency 4—chiasmal injury 2—paramedian infarct (1 death) 1—carotid radiation injury 1—memory loss 1—new onset diabetes insipidus | |
Radioisotope R186 | Derrey (2008) [30] | 42 (11) | 38.7 (5–85) | 0.44 | 0.44 | 3.6 (0.7–12.3) | NR | 3—new visual field cut or acuity decline 2—septic meningitis 2—chemical meningitis 2—central hyperthermia 1—intracranial hypertension 1—memory loss |
Bleomycin | Hader (2000) [40] | 9—all paeds | 8.4 (2.5–14) | 0.14 | 0.71 | 3 (0.5–5) | NR | 2—transient headaches and fever 1—panhypopituitarism |
Park (2002) [44] | 10 (5) | 30.2 (3–65) | NR | NR | 2.8 (1–6.6) | NR | 1—visual disturbance 1—cerebellar infarction/death 1—hypersomnia/memory loss 1—bedridden 1—mental impairment | |
Mottolesseb (2005) [43] | 24—all paeds | NR (6–16) | 0.5 | 0.25 | 6.7 (1–14) | NR | 11—endocrine deficiency 4—visual decline 3—new onset of diabetes insipidus 1—blindness (after toxic dose) | |
Takahashib (2005) [45] | 11—all paeds | NR (2–14) | 0.27 | 0.64 | NR (3–16) | NR | 1—hypothalamic insufficiency/death | |
Hukinb (2007) [42] | 17—all paeds | 6 (1–14) | 0.29 | 0.35 | 5 (0.5–10.2) | 0.7 (0.3–6.1)a | 2—panhypopituitarism 1—cranial nerve palsies and hemiparesis 1—decreased LOC 1—precocious puberty | |
Kim (2007) [46] | 11 (5) | 27.9 (3–67) | 0.36 | 0.64 | 2.6 (0.8–6.6) | 1.4 (0.5–3) | 3—transient headache, vomiting and elevation of body temperature | |
Hsu (2009) [41] | 9—all paeds | 7.8 (3.3–11.8) | NR | NR | 3.7 (0.8–6.6) | 2.4 (0.2–6.6) | NR |
Beta-Emitting Radioisotopes
Over the last 40 years several radioisotopes, particularly phosphorus32, yttrium90 and rhenium186 have been reported to show efficacy as intracystic therapy in craniopharyngioma [23–26]. The use of these agents is, however, now diminished, limited to a select number of global centres with the appropriate equipment and experience in administration [27–29].
Of the small clinical studies published, no particular radioisotope has produced consistently superior results compared to that of other agents; randomised comparison studies are lacking. It could be that the choice of β-emitting radionucleotide used is influenced by accessibility, since phosphorus32 is the only isotope licensed for this purpose in the USA, whereas phosphorus32, yttrium90 and rhenium186 are available across certain institutions in Europe and East Asia [27–30]. Likewise, the radio-physical attributes of the different radionucleotides may determine preference. For instance, phosphorus32 has a longer half-life, emits less energy with a lower resulting tissue penetration than other isotopes [31]. However, yttrium90 is favoured by other groups because of its paradoxical higher dose release and penetration over a shorter time period [32].
While tumour responses following administration of β-emitting radionucleotides have been reported (Table 1), a definition of the degree of such a response has not been standardised for use across these craniopharyngioma studies, therein leading to potential bias. The durability of such responses is also often lacking and limited to the cystic portion receiving therapy without an effect on any neighbouring cysts or solid tumour components if present [14, 27, 33]. In addition, the dose of isotope used between patients is not consistent, reflecting dose adjustments to match treated cyst volume. There is evidence that cyst volumes exceeding 100 ml will not respond well to this therapy, requiring higher dose and potential increased toxicity [21, 30, 34]. Indeed, visual, endocrine, vascular and infective complications have been reported in as many as 30% of exposed cohorts, sadly including fatalities following certain cases [25–31, 33, 35]. Prospective, randomised-controlled evaluations of efficacy are lacking.
Bleomycin
In 1974, the antitumour antibiotic bleomycin was reported to demonstrate in vitro cytotoxicity against cultured craniopharyngioma cells [36]. This finding, in conjunction with the more widespread clinical availability of bleomycin compared to β-emitting radionucleotides, made the chemotherapeutic agent an attractive alternative candidate for use as intracystic therapy.
Over the following 35 years, several case reports [37–39] and retrospective institutional case series reported the efficacy of bleomycin in inducing sustained craniopharyngioma cyst shrinkage for paediatric patients [40–52] (Table 1). However, as was the case for β-emitting radionucleotides, bleomycin did not appear to influence extrinsic craniopharyngioma cyst or solid component growth [14, 46]. The retrospective analyses undertaken are hindered by small cohort sizes, variable definitions of disease response and inconsistent drug dosing and scheduling. Indeed total bleomycin doses administered to patients ranged from 14.5 to 185 mg in one treatment cycle [44, 46, 50, 53]. Moreover, whilst bleomycin appeared to be generally well tolerated with side effects limited typically to headaches, pyrexia and emesis, concerns of significant toxicity were voiced, likely related to drug leakage from the cyst into surrounding brain structures. Post-therapeutic seizures, hemiparesis [42, 44, 53, 54], panhypopituitarism [40, 42], hypothalamic injuries [44, 55], blindness [52, 54] and mortality [45, 56] were all attributed to bleomycin leakage, in some instances despite verification of correct catheter placement.
In 2012 and 2014, two Cochrane systematic literature reviews of intracystic bleomycin use in childhood cystic craniopharyngioma failed to identify any randomised-controlled trials in which the only differing factor was the instillation of bleomycin [57, 58]. As a result, no conclusion on its efficacy could be drawn and the Cochrane review team was unable to recommend bleomycin as a legitimate treatment in this setting. This finding, together with concerns regarding its toxicity following administration [55], has now rendered intracystic bleomycin obsolete as a therapeutic option for cystic craniopharyngiomas.
Intracystic Interferon-alpha: Biological Rationale for Use
Craniopharyngiomas are thought to arise from rests of pharyngeal epithelium during embryonic development of Rathke’s pouch [59]. The secretive squamous epithelium in turn contributes to the formation of cysts containing fluid, epithelial cells and cholesterol-rich crystals [39, 60]. To date, little is known about the biological composition of the cystic fluid extracted from adamantinomatous craniopharyngiomas [61–67] but recent proteomic work has detected an increase in the concentration of anti-inflammatory peptides called alpha-defensins in cyst fluid from untreated children [60]. Explanations for the presence of these alpha-defensins remain elusive, but hypotheses include involvement of the innate immune response system [60], a proposal reinforced by evidence of increased immunoglobulin production in craniopharyngioma cyst fluid [63] and the association of pro-inflammatory Natural Killer (NK) cells with craniopharyngiomas [67].
Interferon-alpha belongs to the large interferon family of cell signalling proteins that are produced and secreted by cells in response to a biological induction from pathogens such as viruses, bacteria and tumour cells [68]. The interferons exert their effect by binding to specific cell receptors, initiating a range of intracellular effects. In tumours, the direct mechanism of action is unclear. It is postulated these actions may include activation of the immunological response system via immune cell activation, cytokine induction and the inhibition of neoplastic vascularisation [69], or direct promotion of cellular differentiation and the inhibition of proliferation through modulating signalling mechanisms such as the JAK-STAT and PI3K pathways [70, 71]. As a consequence, interferon therapy has been used for a range of haematological and solid cancers [72–77].
One such malignancy where interferon-alpha has demonstrated clinical activity is squamous cell carcinoma of the skin [77–79]. Since squamous cell carcinomas and cystic craniopharyngiomas share the same cell of origin, clinical analyses were developed to investigate a potential therapeutic role for this drug in craniopharyngiomas.
A Phase II study of subcutaneous interferon-alpha injections in 12 evaluable patients (aged 4.2–19.8 years) with progressive or recurrent craniopharyngiomas revealed radiological improvement in three patients (25%). Therapy involved daily injections at a dose of 8 million units/m2 for 16 weeks, followed by the same dose three times per week for an additional 32 weeks. All 12 patients developed a transient flu-like illness while seven children required dose reduction or temporary stoppage of the drug due to toxicity [80]. A further, smaller study of five children (aged 9–15 years) with recurrent craniopharyngiomas evaluated the weekly, subcutaneous administration of pegylated interferon-alpha with the theory that the amended formulation would enable a sustained drug exposure, thereby improving efficacy [81]. Four patients (80%) had protracted partial or complete responses to treatment with minimal toxicity. This experience prompted a prospective phase II trial by the Paediatric Brain Tumour Consortium which is still ongoing (ClinicalTrials.gov Identifier: NCT01964300).
These results lend credence to a potential role for interferon-alpha as an intra-lesional therapeutic agent in cystic craniopharyngioma. Moreover, whilst concern surrounding the toxicity from CSF leakage of intracystic β-emitting radionucleotides and bleomycin often precluded their use in this setting, interferon-alpha has been used successfully when administered as intrathecal therapy for subacute sclerosing panencephalitis and neoplastic meningitis [82, 83], suggesting potentially less risk if leakage into surrounding CSF spaces and tissue parenchyma was to occur.
Studies evaluating interferon-alpha as an intracystic therapy in childhood craniopharyngioma have been performed and are described following an overview of the administration schedule that has been typically followed.
Administration of Intracystic Interferon-alpha
On overview of the most widely used administration schedule used in recent clinical studies is shown in Table 2 [14, 84–86]. Two weeks after insertion of the intracystic catheter, a permeability imaging study (either a CT or MRI brain with contrast injection and saline flush into the catheter) is recommended to ensure the correct position of the catheter tip in the cyst (Fig. 2), establish an approximate calculation of cyst volume and to confirm no leakage of contrast occurs from the catheter following instillation. Baseline pituitary functioning and visual assessment is recommended before commencing therapy.
Table 2
Schedule for administration of intracystic interferon-alpha-2b
Drug | Route | Dose | Weeks | Days |
|---|---|---|---|---|
Interferon-alpha-2b
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|