19 Adjuvant Systemic Therapy
Types of Breast Cancer
The recognized histologic subtypes of breast cancer are reviewed in Chapter 2. Invasive cancers can be divided into biologically diverse histologies, although within most histologies biologic aggressiveness ranges from indolent to aggressive and is traditionally expressed as histologic grade (grades 1–3) and proliferative thrust (e.g., S-phase fraction, Ki-67, or MIB-1). Certain histologies such as tubular, “typical” medullary, papillary (non-micro type), and mucinous carcinomas are usually low-grade cancers. Breast cancer is also divided into three general biologic subsets, as defined by estrogen receptors (ER) and progesterone receptors (PR) and HER2 expression (HER2): (1) ER-positive or PR-positive; (2) HER2-positive; and (3) triple-negative (negative for ER, PR, and HER2).
Molecular Subtypes of Breast Cancer
Perou, Sorlie, and colleagues1 set off a paradigm shift in our thinking about breast cancer in their seminal paper describing the molecular portraits of breast cancer by gene expression array. Subsequent papers using both similar and dissimilar techniques have generally confirmed that there appear to be a limited number of biologically distinct subtypes of breast cancer—the most common of which are ER-positive low proliferation (luminal A), ER-positive high proliferation (luminal B), HER2-positive, and triple negative (basal). This chapter is organized with recognition of the interplay between the molecular and clinical classifications. A discussion of adjuvant therapy must balance the risks of tumor recurrence and subsequent mortality with the benefits and risks of the various therapies that can be offered.
Risk of Breast Cancer Recurrence and Mortality
The size of the primary tumor (T), nodal involvement (N), and presence of metastases (M) are the three main clinical criteria for determining stage and prognosis of breast cancer. Other factors contribute to prognosis to a lesser degree. Tumor grade clearly influences prognosis: grade 1 tumors exhibit less propensity for regional and disseminated metastases compared with grade 3 tumors. Unfortunately, pathologists have only about a 40% to 60% agreement when assigning grade. Proliferative thrust as measured by Ki-67 or other nuclear protein markers inversely correlates with outcome. Lymphovascular invasion also predicts increased rates of local-regional recurrence, nodal involvement, and distant metastasis, although limited sampling of the tumor specimen may lead to underestimation of the usefulness of this marker. Untreated, ER-positive and ER-negative tumors have similar 10-year rates of recurrence, although ERpositive tumors tend to be more indolent in their natural history. HER2-positive as well as PR low-proliferation tumors are more aggressive biologically and tend to have a shorter time to recurrence and a greater rate of recurrence.
Not infrequently, the constellation of traditional prognostic factors provides a mixed message. Several newer methods attempt to integrate biology with stage in an effort to improve prognostication. Adjuvant! Online is a Web-based computerized modeling program developed by Dr. Peter Ravdin. It integrates stage (T and N), ER status, and grade to provide estimates for recurrence risk and mortality in patients who are untreated and/or treated with different types of chemotherapy regimens and/or hormonal therapies.2 This program has proved to be a useful aid for many oncologists during the discussion of adjuvant therapy with newly diagnosed patients. One of its beneficial features is the recognition that patients at various ages have different competing risks for mortality. These considerations may be particularly relevant for older persons who are trying to decide whether a potentially toxic intervention might be worth pursuing. It has proved to be quite accurate in predicting outcomes of a population of patients,3 but it has been less accurate in predicting the outcome of individual patients.4 The current version does not yet account for HER2 status or treatment with trastuzumab.
Recently, two methods of evaluating multigene expression have been approved by the Food and Drug Administration (FDA) for use in early-stage breast cancer. The more mature test is the Oncotype Dx assay. It has been approved for use in newly diagnosed patients presenting with ER-positive node-negative breast cancer.5,6 The other test, Mammaprint, has been approved as a prognostic test for a more diverse population of node-negative, early-stage breast cancers.4,7 Oncotype Dx is a commercial assay measuring mRNA levels using quantitative reverse transcription-polymerase chain reaction (RT-PCR) from paraffinembedded formalin-fixed tissues. Sixteen cancer-related genes, including ER, HER2, proliferation genes, and invasion signaling genes, are measured along with five housekeeping genes that serve to normalize the raw data. Validation is extensive and the reproducibility of the assay appears to be excellent. Based on the National Surgical Breast and Bowel Project (NSABP) B-14 and B-20 studies, the recurrence score is a continuous variable ranging from 1 to 100 and provides a prediction for the residual risk of recurrence at 10 years’ follow-up, assuming that the patient will have taken 5 years of tamoxifen.5,6 The level of ER mRNA expression appears to correlate well with the degree of benefit from tamoxifen, and the level of PR mRNA expression appears to correlate inversely with the biologic aggressiveness of the breast cancer. Size remains a weak independent prognostic factor, not accounted for in the recurrence score. HER2-positive tumors are underrepresented in the sample. The test has not yet been validated in node-positive breast cancers. Mammaprint measures the mRNA of 70 genes using fresh tissue and a cDNA chip platform. It dichotomizes the breast cancer population into “good risk” and “poor risk.” This assay has been validated in a more heterogeneous set of patients and treatments than Oncotype Dx has and thus does not provide specific prognostic or predictive information (e.g., response to therapy). Both of these assays are integrated into large ongoing clinical trials that are reviewed later in this chapter.
Adjuvant Chemotherapy
Principles and Overview
With over 20 years of follow-up, adjuvant chemotherapy for breast cancer has been shown to reduce the risk of metastatic recurrence and to increase overall survival. With the gradual evolution of clinical trials from small to large, from broad eligibility to more narrowly defined disease processes, and from aggressive biology to more indolent tumors, the incremental benefits of adjuvant chemotherapy have been demonstrated in essentially all types and stages of breast cancer. It is clear from individual studies and from the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) meta-analyses8 that combination therapy is more effective than single-agent therapy, that anthracycline-based chemotherapy appears superior to non-anthracycline regimens, that taxanes appear to add to anthracycline-based therapies, that maintenance chemotherapy beyond six or eight cycles does not increase survival, and that the targeted therapies (antiestrogen and anti-HER2) add significantly to chemotherapy when the targets are present in the tumor.
Standards of Care
Low-Aggressive Regimens
In the United States, AC (doxorubicin and cyclophosphamide) given intravenously every 3 weeks for four cycles largely supplanted CMF therapy after two NSABP trials demonstrated its equivalence to CMF. Most medical oncologists and patients opted for the shorter AC course. Elsewhere in the world, FEC (5-fluorouracil, epirubicin, and cyclophosphamide) has been the typical standard of care using either the Canadian MA.5 or, more typically, the French regimen.9,10
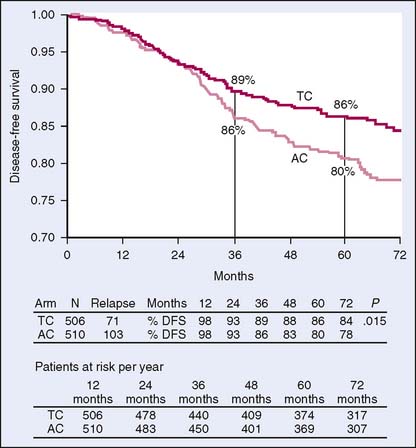
Recently, Jones and colleagues11 reported the 5-year results of a randomized trial comparing the standard AC regimen with TC (docetaxel, cyclophosphamide) for four cycles. This trial demonstrated clear superiority of the TC regimen regarding progression-free survival and overall survival, with a 33% reduction in risk of recurrence (Fig. 19-1). Notably, TC therapy had greater neuropathy and low-grade docetaxel-associated toxicities, but did not have associated cardiac toxicity nor (to date) significant leukemic risks.
High-Aggressive Regimens
In a paper summarizing the 20-year sequence of adjuvant trials in the Cancer and Leukemia Group B (CALGB) for node-positive breast cancer, Berry and colleagues12 highlight the incremental benefits accrued from dose optimization of (F)AC, the addition of paclitaxel to AC, and the impact of dose density on three-drug regimens. Allowing for cross-trial comparisons and historical controls, the sum total resulted in a doubling of progression-free survival in a similar population of patients. Through such sequences of randomized controlled trials, current standards of care such as adjuvant dose-dense AC → T have been established.
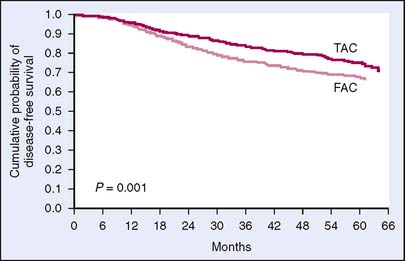
High-aggressive regimens have been supported by several studies. TAC (docetaxel, doxorubicin, and cyclophosphamide) given for six cycles once every 3 weeks became one of the options for node-positive breast cancer after it was associated with a 17% reduction in risk of recurrence compared with FAC in the Breast Cancer International Research Group (BCIRG) 001 trial13 (Fig. 19-2). The PACS 01 (phosphofurin acidic cluster sorting protein 1) trial compared six cycles of FEC chemotherapy with three cycles of FEC followed by three cycles of docetaxel.14 The cross-over arm with docetaxel showed a 14% reduction in risk of recurrence compared with FEC alone. The benefit of docetaxel was also confirmed in the neoadjuvant trial NSABP B-27, although no survival advantage has yet been documented in this study.
Chemotherapy Integration with Antiendocrine Therapy
Tamoxifen is used sequentially both with chemotherapy and radiation. The sequential treat ment is largely based on in vitro studies, showing a decrease in sensitivity of cultured breast cancer cells to radiation when concurrently treated with tamoxifen. It is further supported by clinical observations of increased pulmonary toxicity with the concurrent approach.15 Chemotherapy integration with antiendocrine therapy has been examined in the SWOG (Southwest Oncology Group) S8814 trial16 (Table 19-1). In this trial, postmenopausal women with ER-positive node-positive breast cancer were randomized to tamoxifen alone (T); cyclophosphamide, doxorubicin, and 5-fluorouracil (CAF) chemotherapy concurrent with tamoxifen (CAFT); or CAF chemotherapy followed by tamoxifen (CAF → T). Although only available in abstract form at this point, the finding that chemotherapy improved survival compared with tamoxifen alone in this population of patients has been influential. Even more important has been the finding that sequential therapy with CAF followed by tamoxifen was superior to the other two groups. A definitive report of this trial will be published shortly in a major journal (Albain KS et al). The mechanism of sub-additive efficacy when combining tamoxifen with CAF is unknown. One possibility is that the reduction of proliferative thrust by reducing estrogenic signaling could reduce chemosensitivity. If so, then concurrent administration of aromatase inhibitors or other endocrine therapies with chemotherapy should not be done, although this hypothesis has not been tested adequately.
Table 19-1 Impact of Adding Chemotherapy to Tamoxifen for Postmenopausal Women with ER-Positive, Node-Positive Breast Cancer According to the Oncotype DX Recurrence Score
| 10-Year Disease-Free Survival Estimates (%) | ||
|---|---|---|
| Tamoxifen (n = 148) | CAF → Tamoxifen (n = 219) | |
| Low recurrence score (<18) | 60 | 64 |
| Intermediate recurrence score (18–30) | 49 | 63 |
| High recurrence score (≥31) | 43 | 55 |
CAF, cyclophosphamide, doxorubicin, and 5-fluorouracil; ER, estrogen receptor.
From Albain K et al. Prognostic value of the Oncotype DX assay in the chemotherapy-based arms of SWOG-8814. Breast Cancer Update 2, 2008. Available at www.breastcancerupdate.com/medonc/2008/2/albain.asp.
Future Directions
Predictors of Response/Benefit to Particular Chemotherapy Agents
Using biologic markers to determine who will benefit from certain agents is an area of significant interest. For example, knowing who will benefit most from anthracyclines is of clinical importance. The presence of the topoisomerase II enzyme (topo II) is required for topoisomerase II inhibitors (e.g., anthracyclines) to be effective. Recent studies reported by the National Cancer Institute of Canada (NCIC) suggest that the high nuclear staining for topo II seen in approximately 25% to 30% of breast cancers predicts a better response to anthracyclines.17 Less convincing are reports examining elevated topo II mRNA levels or topo II gene amplification. Topo II gene is amplified in up to 35% of HER2-amplified breast cancers, but it is relatively rare in the HER2-negative breast cancers. The presence of HER2 amplification appears to be associated with a greater benefit to anthracycline-containing regimens, but this is not as clearly explained by topo II changes.
A number of predictors of sensitivity and resistance to taxanes have been published using a battery of immunohistochemistry probes or gene expression arrays.18,19 These have been variably validated in neoadjuvant chemotherapy trials, but have not yet been tested in definitive prospective trials. New taxanes and taxoids, such as nab-paclitaxel and the epotheliones, will be or are already being tested in the adjuvant setting.
Advances in pharmacogenomics and the ability to measure global expression of single nucleotide polymorphisms (SNPs) will contribute to personalized medicine. Ambrosone and colleagues20 recently reported that the presence of SNPs associated with high levels of myeloperoxidase, and thus heightened ability to form free radicals and oxidative reactive intermediates, appears to be associated with a greater benefit from cyclophosphamide-containing chemotherapy. Along similar lines, population heterogeneity with respect to SNPs for genes regulating tamoxifen metabolism and activation may play a significant role in determining benefit from tamoxifen. These studies cannot be performed without rigorous and comprehensive tissue banking of suitable specimens.
The TAILORx trial will enroll 11,000 women with ER-positive node-negative breast cancers and categorize them according to Oncotype Dx recurrence score.21 Approximately 30% are expected to have low recurrence scores (less than 11) and to have antiestrogen therapy alone. The approximately 20% expected to have high recurrence scores (more than 25) will receive chemotherapy followed by endocrine therapy. The remainder with intermediate recurrence scores will be randomized to receive antiestrogen therapy with or without chemotherapy. By measuring progression-free survival, it is hoped that up to 50% of women with intermediate recurrence scores will be found not to require chemotherapy.
The MINDACT study, based on the Mammaprint assay, will assign about 8000 women with stage I node-negative disease (including ER-negative) to good or bad prognosis by both standard clinical features and by gene expression profiling.22 Those with discordance between the clinical prognosis and the gene expression prognosis will be randomized to receive therapy based on either the clinical prognosis or the gene expression prognosis. This will help determine whether the gene expression profile predicts biologic behavior and response to therapy better than standard clinical parameters.
Within the triple-negative phenogenotype, there are identifiable sub-subtypes of breast cancer. For example, a group of these are either the BRCA1 mutated tumors or tumors with loss of BRCA1 protein expression. These tumors appear to be exquisitely sensitive to alkylating agents and radiation therapy because of defects in DNA repair pathways.23 Poly (ADP-ribose) polymerase (PARP) inhibition may increase this sensitivity.24 Others appear to have increased activation of the androgen receptor pathway genes.25 It remains to be seen whether anti-ndrogen receptor therapy will have activity in some triple-negative tumors. Undoubtedly, other sub-subtypes will be identified on the basis of increased epidermal growth factor receptor (EGFR) expression, sensitivity to Src or c-Met inhibition, or other characteristics. Once confirmed in metastatic disease trials, these concepts will rapidly disseminate to adjuvant and/or neoadjuvant settings.
Another concept that may become a standard of care in the future is the use of bisphosphonates as adjuvant therapy for breast cancer. The bisphosphonates inhibit osteoclast function and are widely used for the treatment and prevention of osteoporosis. They have also proved to decrease the rate of progression of breast and other cancers involving bone and to decrease rates of fracture and pain from metastases. In model systems, breast cancer cells home to bone marrow spaces, activate osteoclasts through parathyroid hormone-related protein (PTHrP) and other mechanisms. Osteoclasts, in turn, release various cytokines such as interleukin-6 (IL-6) and tumor growth factor-β (TGF-β) from bone matrix, which then support breast cancer cell growth and survival. Interruption of these pathways can make bone a less friendly environment for breast cancer cells to grow.26
Three clodronate adjuvant trials have been reported; two demonstrated a small survival advantage with 10-year follow-up.27–30 A fourth trial (NSABP B-34) has completed accrual, but has not reached maturity. The AZURE trial, comparing zoledronate with placebo, has likewise completed accrual. SWOG S0307 is an intergroup trial randomizing patients to three different bisphosphonates for 3 years. This trial is asking the question whether more potent aminobisphosphonates (ibandronate and zoledronate) would be more effective than clodronate (a weak bisphosphonate).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree