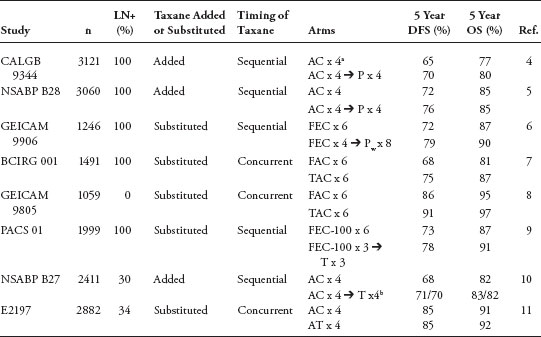
Adjuvant Chemotherapy for Breast Cancer: Updates and New Perspectives The adjuvant breast cancer treatment landscape has undergone notable changes in recent decades. After the successful development of early polychemotherapy regimens, incremental gains have been achieved with the incorporation of anthracyclines and subsequently taxanes into adjuvant systemic strategies. Many investigators have since aimed to optimize modern chemotherapy regimens by altering sequencing, scheduling, and/or drug delivery. Furthermore, insights into breast cancer subtypes as defined by molecular profiling, have permitted tailoring of specific treatment recommendations to the biology of an individual’s tumor and the successful development of so-called targeted therapies. In fact, commercially available gene profiling tools are increasingly used in clinical practice to identify subgroups of patients who are likely to derive benefit from specific systemic strategies. Consequently, modern clinical trial designs are increasingly focused on evaluating the efficacy of specific systemic therapy strategies in specific subgroups of patients with breast cancer. It is hoped that these innovations will ultimately translate into improved outcomes, and ideally cure, for affected individuals. Keywords: breast cancer, adjuvant, chemotherapy, anthracyclines, sequence, schedule, gene profiling Breast cancer is a global public health issue. More than one million new cases are diagnosed worldwide, and approximately 200,000 new cases are diagnosed in the United States each year (1,2). In the developed world, most newly diagnosed breast cancers are early stage at diagnosis and, therefore, potentially curable (2). However, despite therapy with curative intent, approximately one third of women with an early-stage diagnosis experience a distant relapse. Because metastatic breast cancer is treatable but not curable, there is a window of opportunity at the time of early-stage diagnosis to maximize the probability of cure. Consequently, investigators strive to optimize early-stage treatment paradigms. Because breast cancer is a heterogenous disease, other notable recent innovations reflect critical insights into the biology of specific subtypes. For example, modern gene profiling techniques often enable clinicians to make adjuvant chemo-therapy recommendations that are tailored to the biology of an individual’s tumor. It is hoped that further adjuvant systemic therapy improvements in conjunction with additional biologic insights will ultimately translate into cure for more women with early-stage breast cancer. Anthracyclines After the first polychemotherapy regimens, such as CMF, proved efficacious after surgery for early-stage breast cancer, investigators aimed to further improve adjuvant therapy. One of the earliest tactics was the incorporation of an anthracycline, either doxorubicin or epirubicin. After multiple studies evaluating conventional strategies versus anthracycline-based strategies were completed, the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) conducted a meta-analysis of data from 194 relevant randomized trials to evaluate the impact of this innovation. The 2005 EBCTCG report confirmed that the incorporation of anthracyclines into adjuvant treatment strategies resulted in an absolute 4% reduction in the risk of recurrence and death at 10 years compared with conventional chemotherapy with CMF (3). Furthermore, anthracycline-containing regimens conferred a 38% decrease in the annual breast cancer death rate for women younger than 50 years and a 20% decrease for women 50 to 69 years of age largely irrespective of tamoxifen use, hormone receptor status, nodal status, or other conventional tumor characteristics. Taxanes Paclitaxel The development of the taxanes, paclitaxel and docetaxel, and their subsequent successful incorporation into adjuvant chemotherapy strategies represents a major therapeutic advance in the management of early-stage breast cancer. Taxanes exert their activity by stabilizing microtubules. After significant benefits were observed with these drugs in the metastatic setting, several large, randomized, adjuvant studies were undertaken. Many investigators endeavored to build on the success of anthracycline-based strategies by administering either concurrent or sequential taxane therapy (Table 1). Three large studies evaluated anthracycline-based chemotherapy with or without paclitaxel, with consistent benefits in favor of paclitaxel administration (4–6). For example, in the Cancer and Leukemia Group B (CALGB) 9344 study, 3,121 women with node-positive early-stage breast cancer were randomized to four cycles of doxorubicin and cyclophosphamide (AC) with or without four subsequent cycles of paclitaxel at 175 mg/m2 (4). The addition of paclitaxel significantly improved the risk of disease recurrence (hazard ratio [HR]0.83, P = .023) and survival (HR 0.82, P = .0064). Notably, this study also addressed the question of dose escalation: doxorubicin was administered at 60, 75, or 90 mg/m2, but no additional benefits were observed beyond the 60 mg/m2 dose. In NSABP B-28, 3,060 women with node-positive breast cancer were randomized to four cycles of AC with or without sequential paclitaxel at 225 mg/m2 (5). The addition of paclitaxel was associated with a reduction in DFS risk (HR 0.83, P = .006) that was comparable with the DFS benefits observed in CALGB 9344. However, unlike CALGB 9344, no OS benefits were observed in NSABP B-28 (5-year OS 85% in both arms). In a smaller multicenter European study, GEICAM 9906, 1,246 women with node-positive breast cancer were randomized to six cycles of fluorouracil, epirubicin, and cyclophosphamide (FEC) or four cycles of FEC followed by eight cycles of weekly paclitaxel at 100 mg/m2 (6). Because of the dose and scheduling differences between the two arms, this was not a pure study of an anthracycline-based regimen with or without paclitaxel. However, results consistent with the other two studies were observed. Specifically, the paclitaxel-containing regimen was associated with significant improvements in 5-year DFS (78.5% vs 72.1%, P = .006). Despite a trend in favor of the taxane-containing arm for OS, no significant differences were observed between the two arms (HR 0.78, P = .110). Selected studies evaluating the addition of a taxane to adjuvant anthracycline regimens LN+, percentage of patients with axillary lymph node involvement. “Taxane added” refers to the use of taxane in the experimental arm in addition to the entire treatment given in the control arm. “Taxane substituted” refers to the replacement of part or all of the control arm by the taxane-containing portion of treatment. AC, doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2. P, paclitaxel 175 mg/m2. FEC, fluorouracil 600 mg/m2, epirubicin 90 mg/m2, and cyclophosphamide 600 mg/m2. Pw, paclitaxel 100 mg/m2 weekly. FAC, fluorouracil 500 mg/m2, doxorubicin 50 mg/m2, and cyclophosphamide 500 mg/m2. TAC, docetaxel 75 mg/m2, doxorubicin 50 mg/m2, and cyclophosphamide 500 mg/m2. FEC-100, fluorouracil 500 mg/m2, epirubicin 100 mg/m2, and cyclophosphamide 500 mg/m2. T, docetaxel 100 mg/m2. AT, doxorubicin 60 mg/m2 and docetaxel 60 mg/m2. aThree doses of doxorubicin were also evaluated in this study: 60 mg/m2, 75 mg/m2, and 100 mg/m2. bDocetaxel was evaluated in 2 cohorts, given before or after definitive surgery. The figures for DFS and OS are given separately for pre- and postoperative docetaxel arms. Docetaxel Numerous adequately powered studies have evaluated the incorporation of docetaxel into adjuvant anthracycline-based chemotherapy strategies (7–10). In BCIRG 001, approximately 1,500 women with node-positive breast cancer were randomized to six cycles of every 3-weekly 5-fluorouracil at 500 mg/m2 with doxorubicin at 50 mg/m2 and cyclophosphamide at 500 mg/m2 (FAC) or six cycles of docetaxel at 75 mg/m2 with doxorubicin at 50 mg/m2 and cyclophosphamide at 500 mg/ m2 (TAC) (7). After a 55-month median follow-up period, the taxane-containing regimen conferred significant improvements in 5-year DFS (75% vs 68%; HR 0.72, P = .001) and OS (87% vs 81%; HR 0.7, P = .008). However, TAC was associated with a significantly increased rate of febrile neutropenia (24.7% vs 2.5%, P < .001). In GEICAM 9805, 1059 women with node-negative breast cancer were randomized to six cycles of every 3-weekly TAC or FAC (8). After a median follow-up of 6 years, benefits were reported for DFS (91% vs 86%, P = .0202), but not OS (97% vs 95%, P = .2677). In PACS 01, 1,999 women with node-positive breast cancer were randomized to six cycles of FEC (a FAC-like regimen containing the anthracycline epirubicin rather than doxorubicin) or three cycles of FEC followed by three cycles of docetaxel (9). The docetaxel-containing regimen was associated with significant DFS (78% vs 73%; HR 0.83, P = .041) and OS (91% vs 87%; HR 0.77, P = .05) benefits. Notably, however, not all studies have demonstrated benefits with combination anthracycline– taxane strategies. In NSABP B-27, for example, women were randomized to preoperative AC with or without pre- or postoperative docetaxel, with no DFS or OS benefits reported in favor of any of the three study arms (10). Similarly, in E2197, 2,882 women with node-positive or “high-risk” node-negative breast cancer were randomized to four cycles of every 3-weekly AC or doxorubicin with docetaxel, with no demonstrated benefits in favor of either strategy (11). Furthermore, more grade 3 febrile neutropenia (26% vs 10%) was observed in the experimental arm. In the TACT study, more than 4,000 women with node-positive or “high-risk” node-negative breast cancer were randomized to eight cycles of FEC or four cycles of FEC followed by four cycles of docetaxel (12). After a median follow-up period of almost 5 years, no differences were observed in favor of either arm—a result that was notably discordant with the PACS 01 results (9). Although cross-study comparisons are discouraged, it is dificult to account for the inconsistent findings across studies. Some possible explanations include dose diferences across studies. For example, the doxorubicin and cyclophosphamide doses administered in the FAC regimen are less than those in conventionally dosed AC. Therefore, it is possible that concurrent docetaxel can supplement the efficacy of anthracyclines, but only when the anthracycline is administered at less than conventional doses. Furthermore, the discordance between the PACS 01 and TACT outcomes indicates that docetaxel may only confer benefits when added to FEC when fewer anthracycline cycles have been administered. Furthermore, low event rates in lower risk populations may also limit interpretability in some studies. To address the discordant results across the adjuvant taxane studies, several pooled analyses have been undertaken. Three large pooled analyses have concluded that taxanes improve DFS by approximately 2% to 3% and OS by approximately 3% to 5%, although notably, significant overlap exists across these studies (13–15). Furthermore, taxane benefit does not appear to be affected by age, menopausal status, number of involved lymph nodes, type of taxane administered, or schedule. It is anticipated that the forthcoming EBCTCG meta-analysis will provide further insights. The Role of Anthracyclines in the Modern Adjuvant Era The successful development of the anthracyclines and their subsequent incorporation into adjuvant strategies represented a notable innovation in early-stage breast cancer management with an associated 16% reduction in the annual risk of death compared with conventional strategies (3). With the subsequent successful development of paclitaxel and docetaxel, anthracyclines and taxanes have become the most active agents in breast cancer. However, because anthracycline administration is associated with a small but significant risk of cardiotoxicity, some have questioned whether anthracycline administration is necessary when a taxane is being administered. This question was explored in US Oncology 9735, the results of which were first presented in 2005 and published in 2009 (16). In this study, approximately 1,000 women with stage I–III breast cancer were randomized to four cycles of AC (60/600 mg/m2) or four cycles of TC (75/600 mg/m2). Notably, approximately half of the patients had node-negative disease. After a 7-year median follow-up period, superior DFS (81% vs 75%; HR 0.74; P = .033) and OS (87% vs 82%; HR 0.69; P = .032) outcomes were reported in favor of the taxane-containing regimen. However, the significance of the US Oncology results in the modern adjuvant era is uncertain. For example, the AC control arm might not be considered a modern strategy given that four cycles of AC followed by four cycles of paclitaxel was demonstrated to yield superior DFS in two studies (4,5) and OS in one study (4) compared with four cycles of AC alone. Furthermore, every 2-weekly (dose dense) AC-T is superior to conventionally scheduled, every 3-weekly AC-T (17,18). Because the performance of TC has not been evaluated against a modern strategy such as dose-dense AC-T, many clinicians continue to favor an adjuvant strategy that incorporates both an anthracycline and taxane, particularly for women with high-risk disease. The Impact of Dose and Scheduling on Adjuvant Chemotherapy Strategies Two strategies designed to optimize tumor cell kill, dose escalation or intensity (whereby the dose of cytotoxic agent delivered at each chemotherapy cycle is optimized) and dose density (whereby the interval between chemotherapy cycles is optimized), have been explored with varying success. In doxorubicin and cyclophosphamide dose-escalation studies, escalation beyond conventional doses has not been shown to increase efficacy (19,20). Furthermore, the ultimate iteration of dose escalation, namely “high-dose” chemotherapy with stem cell support, did not prove beneficial in randomized phase III studies (21,22). Dose-dense strategies, on the other hand, have yielded favorable results. The rationale for the dose-dense approach is based on the Norton-Simon model, which uses mathematical analyses to predict growth patterns in tumor systems (23). This model predicts that decreasing the interval between treatment cycles optimizes the fixed cell kill with each cycle, thereby improving the overall impact of therapy. This hypothesis was tested in CALGB 9741, a large, randomized, phase III study of sequential doxorubicin, paclitaxel, and cyclophosphamide (A-T-C) or concurrent AC followed by paclitaxel (AC-T) (17,18
 ABSTRACT
ABSTRACT
 BACKGROUND
BACKGROUND
 INNOVATIONS IN ADJUVANT SYSTEMIC THERAPY
INNOVATIONS IN ADJUVANT SYSTEMIC THERAPY
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


