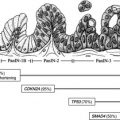
Pancreas adenocarcinoma is an aggressive malignancy. The risk of recurrence remains high even for patients with localized disease undergoing surgical resection. Adjuvant systemic therapy has demonstrated the ability to reduce the risk of recurrence and prolong survival. Determination of optimal adjuvant treatment, systemic therapy, and/or combinations to further improve recurrence rates and overall survival are still needed. Neoadjuvant therapy represents an alternative emerging paradigm of investigation with several theoretic advantages over adjuvant therapy. This article summarizes the major adjuvant and neoadjuvant studies for pancreas adenocarcinoma and highlights key areas of ongoing investigation.
Key points
- •
Current international standards of care for adjuvant therapy for pancreas adenocarcinoma consist of 6 months of gemcitabine or 5-fluorouracil with leucovorin.
- •
Erlotinib does not provide additional benefit in the treatment of patients with resected or locally advanced pancreas adenocarcinoma.
- •
Neoadjuvant therapy provides theoretic advantages over standard adjuvant therapy including treatment of distant micrometastases, assessment of tumor response to treatment, and better selection of patients most appropriate for surgery.
- •
The use of combination cytotoxic therapy, targeted agents, incorporation of chemoradiation, and immunotherapy all represent approaches under active investigation for adjuvant and neoadjuvant treatment of pancreas adenocarcinoma.
Introduction
Patients diagnosed with pancreas adenocarcinoma have a poor prognosis with 5-year overall survival (OS) rates estimated to be 6%. Although surgery for patients with localized resectable pancreas adenocarcinoma remains a potential curative modality, the risk of relapse remains substantial with local-regional recurrence rates from 50% to 80% and systemic recurrence rates of greater than 70%. The value of adjuvant systemic therapy has been clearly established in terms of reducing the risk of recurrence and prolonging survival, albeit the risk of recurrence remains significant. The determination of optimal adjuvant treatment modalities between the types of systemic therapy used, combined chemotherapy and radiation, and chemoradiation plus chemotherapy along with whether to pursue an adjuvant or neoadjuvant strategy remain areas of active investigation. Furthermore, neoadjuvant therapy provides an emerging paradigm with several theoretic advantages over adjuvant therapy. Although not based on randomized data but rather consensus opinion, neoadjuvant therapy before surgery is an emerging paradigm for patients with borderline resectable pancreas cancer.
Introduction
Patients diagnosed with pancreas adenocarcinoma have a poor prognosis with 5-year overall survival (OS) rates estimated to be 6%. Although surgery for patients with localized resectable pancreas adenocarcinoma remains a potential curative modality, the risk of relapse remains substantial with local-regional recurrence rates from 50% to 80% and systemic recurrence rates of greater than 70%. The value of adjuvant systemic therapy has been clearly established in terms of reducing the risk of recurrence and prolonging survival, albeit the risk of recurrence remains significant. The determination of optimal adjuvant treatment modalities between the types of systemic therapy used, combined chemotherapy and radiation, and chemoradiation plus chemotherapy along with whether to pursue an adjuvant or neoadjuvant strategy remain areas of active investigation. Furthermore, neoadjuvant therapy provides an emerging paradigm with several theoretic advantages over adjuvant therapy. Although not based on randomized data but rather consensus opinion, neoadjuvant therapy before surgery is an emerging paradigm for patients with borderline resectable pancreas cancer.
Historic adjuvant systemic trials
The major historic phase III trials of adjuvant therapy in pancreas adenocarcinoma are listed in Table 1 . One of the earliest phase III trials was the Gastrointestinal Tumor Study Group (GITSG) trial. Forty-three evaluable patients with surgically resected pancreas adenocarcinoma were randomized to receive adjuvant treatment with 5-fluorouracil (5-FU) concurrent with a split-course of radiation versus observation. Median survival was found to be significantly longer for the adjuvant treatment group at 20 months compared with 11 months for the observation group ( P = .035). Although the GITSG study has been used by some as a basis for 5-FU-based chemoradiation in the adjuvant setting, other studies have challenged the value of chemoradiation inferring the benefit observed came from prolonged systemic therapy. The EORTC 40891 (European Organization for Research and Treatment of Cancer) trial was a large multicenter phase III study of patients with resected pancreatic head cancer and periampullary tumors. Patients were randomized to either observation or postoperative chemoradiation with short-course infusional 5-FU with concurrent split-course radiation. A total of 120 patients (approximately >50% of the total study population) with resected pancreatic head cancer were evaluated as part of this study and the long-term follow-up analysis showed no significant difference in survival between the treatment and observation groups even when only the pancreatic head cancer group was evaluated, although a nonstatistically significant trend was in favor of adjuvant therapy.
| Trial | Patients | Treatment Arms | Median Disease-Free Survival (Months) | Median Overall Survival (Months) |
|---|---|---|---|---|
| GITSG-9173 | 43 |
| 2 y survival: 48% vs 14% | 21 vs 11 ( P = .035) |
| EORTC-40891 | 218 |
| 17.4 vs 16 ( P = .643) | 24.5 vs 19 ( P = .208) |
| ESPAC-1 | 289 |
| Chemo vs no chemo: 15.3 vs 9.4 ( P = .02) CRT vs no CRT: 10.7 vs 15.2 ( P = .04) | Chemo vs no chemo: 20.1 vs 15.5 ( P = .009) CRT vs no CRT: 15.9 vs 17.9 ( P = .05) |
| CONKO-001 | 368 |
| 13.4 vs 6.9 ( P < .001) | 22.8 vs 20.2 ( P = .005) |
| RTOG 9704 | 451 |
| No difference, NA | 20.5 vs 16.9 ( P = .09) |
| ESPAC-3 (v2) | 1088 |
| 14.3 vs 14.1 ( P = .53) | 23.6 vs 23.0 ( P = .39) |
| JASPAC-01 | 385 |
| Relapse-free survival: 11.2 vs 23.2 ( P < .0001) | 25.5 vs 46.3 ( P < .0001) |
Along with EORTC 40891, the ESPAC-1 (European Study Group of Pancreatic Cancer) trial further challenged the value of chemoradiation and rather suggested that chemotherapy alone provided a survival benefit in the adjuvant setting. This study used a complex 2 × 2 factorial study design to randomize patients undergoing curative resection for pancreas adenocarcinoma into four treatment arms: (1) chemoradiation consisting of an intravenous bolus of 5-FU with split-course radiation, (2) chemotherapy consisting of an intravenous bolus of leucovorin followed by intravenous bolus of 5-FU for a total of 6 months of therapy, (3) combination therapy consisting of chemoradiation followed by chemotherapy as described previously, or (4) observation. A total of 289 patients underwent randomization with results showing the 5-year survival rate was 21% among patients treated with chemotherapy versus 8% among patients not treated with chemotherapy ( P = .009). In addition, the estimated 5-year survival rate was 10% for patients treated with chemoradiation compared with 20% for patients who did not receive chemoradiation ( P = .05). These results ultimately lead to a path away from using chemoradiation in Europe and beyond in favor of systemic chemotherapy alone as the main adjuvant treatment choice for resected pancreas adenocarcinoma. However, in North America, debate over the radiation techniques used in the EORTC 40891 and ESPAC-1 studies underpin the ongoing controversies over the value of chemoradiation in the adjuvant setting, which continues to remain an area of ongoing investigation and is being evaluated as part of RTOG 0848 ( NCT01013549 ; Radiation Therapy Oncology Group).
Although ESPAC-1 demonstrated the benefit of adjuvant systemic chemotherapy alone in pancreas adenocarcinoma with intravenous 5-FU, the CONKO-001 (Charite Onkologie 001) trial was designed to compare adjuvant intravenous gemcitabine with observation alone in patients undergoing curative resection for pancreatic cancer. A total of 368 patients were randomized to either observation or to receive 6 months of gemcitabine. The primary end point was disease-free survival (DFS) and the key result of the study was a significant DFS advantage of 13.4 months in the treatment group versus 6.7 months in the observation group ( P < .001). In addition, patients in the treatment group were found to have significantly prolonged OS compared with those being observed ( P = .01). These findings from the CONKO-001 therefore provided strong level 1 evidence supporting the use of gemcitabine as a standard chemotherapy agent in the adjuvant setting.
In addition to CONKO-001, the RTOG 9704 study was a US-based phase III trial to determine if the addition of gemcitabine to adjuvant fluorouracil-based chemoradiation improved survival in patients with resected pancreatic adenocarcinoma. A total of 451 patients were enrolled and randomized to chemotherapy with either 5-FU or gemcitabine for 3 weeks before chemoradiation therapy and for 12 weeks following chemoradiation therapy. With a key primary end point of OS for patients with pancreatic head tumors, the investigators reported a median survival of 20.5 months in the gemcitabine group versus a median survival of 16.9 months in the 5-FU group ( P = .09). Although not statistically significant, the authors concluded that the addition of gemcitabine given before and after chemoradiation was associated with a survival trend in the adjuvant setting. Furthermore, stratification of postresctional carbohydrate antigen (CA) 19-9 demonstrated the use of CA 19-9 as a significant predictor of survival in patients treated with adjuvant chemoradiation. As a result, CA 19-9 is now frequently being used as a key eligibility criteria or stratification factor in many pancreas cancer clinical trials.
The ESPAC investigators also began to build on results from their ESPAC-1 trial. The ESPAC-3 (v2) trial ultimately accrued a total of 1088 patients who received either 5-FU plus leucovorin or gemcitabine chemotherapy for 6 months in the adjuvant setting. The final results revealed equivalency between the two different chemotherapy agents with a median survival of 23.0 months for patients treated with 5-FU plus leucovorin and 23.6 months for those patients treated with gemcitabine ( P = .39). The study also reported that 14% of patients treated with 5-FU plus leucovorin developed serious (> grade 3) treatment-related adverse events compared with 7.5% of patients treated with gemcitabine ( P < .001). Based on these results, the use of gemcitabine chemotherapy alone became favored as the predominant therapy in the adjuvant setting. However, these data also provide support for the use of 5-FU/leucovorin in settings where patients may be at risk or develop serious complications to gemcitabine.
Although gemcitabine chemotherapy alone is often recommended as a current standard adjuvant chemotherapy for resected pancreas adenocarcinoma, many trials under investigation are focused on adding either different chemotherapy or biologic agents to gemcitabine or the use of other agents. Recently, preliminary results of the 385-patient JASPAC-01 (Japan Adjuvant Study Group of Pancreatic Cancer) trial suggested that S-1 (an oral fluoropyrimidine) seems to be not only noninferior to gemcitabine but was also superior to gemcitabine in the adjuvant setting for the Japanese patient subpopulation. Although the initial results are impressive, it is unclear if the survival benefit with adjuvant S-1 will translate to a broader population because white persons receiving S-1 have been known to develop more severe gastrointestinal toxicities and therefore lower doses of S-1 may be required in a broader patient population, which may diminish the overall efficacy of the drug. Future results and publication of this trial are awaited.
Current and future areas of investigation in adjuvant therapy
Currently, several approaches being explored in the adjuvant setting to improve outcomes include evaluating (1) the role of combination cytotoxic therapy, (2) the role of the addition of a targeted agent, (3) the role of chemoradiation therapy, and (4) the role of immunotherapeutic approaches. These selected areas of investigation are listed in Table 2 and are described next.
| Trial | Patients | Trial Design | Primary End Point |
|---|---|---|---|
| ESPAC-4 (ISRCTN96397434) | 1080 | Gemcitabine + capecitabine vs gemcitabine | Overall survival |
| CONKO-005 (DRKS00000247) | 436 | Gemcitabine + erlotinib vs gemcitabine | Equivalent median 11.6 mo disease-free survival between groups reported ( P = .291) |
| RTOG 0848 ( NCT01013649 ) | 952 | First randomization: gemcitabine + erlotinib vs gemcitabine (now discontinued) Second randomization: chemoradiation vs no chemoradiation | Overall survival |
| PRODIGE/ACCORD 24 ( NCT01526135 ) | 490 | Gemcitabine vs FOLFIRINOX | Disease-free survival at 3 y |
| APACT ABI-007-PANC-003 ( NCT01964430 ) | 800 | Gemcitabine vs gemcitabine + nab-paclitaxel | Disease-free survival |
| NewLink Genetics Corporation ( NCT01072981 ) | 722 | Gemcitabine (+/- chemoradiation) +/- hyperacute immunotherapy | Overall survival |
In terms of combination cytotoxic therapies, ESPAC-4 (ISRCTN96397434) is a large randomized phase III trial comparing the addition of capecitabine plus gemcitabine to gemcitabine. The study is powered for OS with a target of 1080 patients. The study completed recruitment in late 2014 and results are awaited. In addition to capecitabine being added to gemcitabine, combination cytotoxic regimens with significant benefit in metastatic pancreas adenocarcinoma, such as FOLFIRINOX (5-FU, leucovorin, irinotecan, oxaliplatin) and the combination of gemcitabine plus nab-paclitaxel, are now currently under investigation in the adjuvant setting. For example, investigators from the PRODIGE group, which conducted the prior FOLFIRINOX study in the metastatic setting, have now developed PRODIGE 24/ACCORD 24 ( NCT01526135 ), which is a phase III trial comparing adjuvant chemotherapy with gemcitabine versus modified FOLFIRINOX (omission of bolus 5-FU) to treat resected pancreatic adenocarcinoma. The estimated enrollment will be 490 patients with the primary outcome being DFS at 3 years. Another key study, ABI-007-PANC-003 (NCT01964430), the APACT trial, will compare the efficacy of nab-paclitaxel in combination with gemcitabine with gemcitabine alone as adjuvant treatment in patients with surgically resected pancreatic adenocarcinoma. This study will recruit 800 patients to evaluate the primary outcome of DFS.
Regarding targeted therapy, erlotinib is the agent that has been most extensively investigated in the adjuvant setting based on positive results reported in the locally advanced and metastatic treatment settings for pancreas adenocarcinoma. The CONKO-005 (DRKS00000247) trial recently reported on the role of gemcitabine plus erlotinib compared with gemcitabine alone in patients with R0 resected pancreas cancer. The primary end point of the study was DFS. A total of 436 patients were accrued and results of this study were just recently presented at a median follow-up of 41 months; there was no significant difference between the two treatment groups in terms of median DFS (11.6 months for both groups; P = .291) and OS (24.6 months for gemcitabine plus erlotinib vs 26.5 months for gemcitabine; P = .406). In addition to CONKO-005, RTOG 0848 ( NCT01013549 ) is an active North American phase III trial also originally designed to evaluate the role of the addition of erlotinib in the adjuvant setting while also attempting to address the value of chemoradiation in the adjuvant setting. The study was originally designed for a target of 952 patients to be randomized to receive gemcitabine or gemcitabine plus erlotinib to complete a total of 6 months of adjuvant systemic therapy. Patients in this study will also undergo restaging after 5 months of chemotherapy and if found to have no recurrence, they will undergo a second randomization to the addition of chemoradiation versus no added therapy. In 2013, results from the LAP-07 phase III trial were presented demonstrating that the addition of radiation did not improve outcomes following 4 months of systemic therapy in patients with locally advanced pancreas adenocarcinoma. In addition, there was no change in survival outcomes for patients in the LAP-07 study who were first randomized to receive gemcitabine alone versus gemcitabine plus erlotinib. Given these findings the RTOG 0848 study has since been amended, where patients will now only undergo one randomization to plus or minus the addition of fluoropyrimidine-based radiation to a single-agent gemcitabine cytotoxic backbone and the randomization to include erlotinib has been discontinued. Based on all of the previously mentioned studies, erlotinib seems to have no significant benefit in the treatment of patients with resected and locally advanced pancreas cancer.
Another area of increasing research interest in the adjuvant treatment of pancreas adenocarcinoma has been the development of the use of vaccinations and broad immunotherapeutic approaches. Although vaccinations targeting KRAS mutations, the telomerase peptide vaccine GV1001, and the allogenic whole cell vaccine GVAX (granulocyte-macrophage colony–stimulating factor gene-transfected tumor cell vaccine) have yielded mixed results from prior studies, vaccine development using the concept of hyperacute rejection may have potential promise moving forward. A vaccine (algenpantucel-L) has been developed using genetically modified pancreas cancer cells with a mouse gene leading to foreign protein expression of α (1,3)-galactosyl (αGal). Pre-existing anti-αGal antibodies then trigger a significant immune response leading to proposed cell destruction of any tumor cells in patients undergoing treatment with this form of immunotherapy. A phase II study evaluating the role of this form of algenpantucel-L immunotherapy in addition to therapy with gemcitabine with 5-FU-based chemoradiation in patients with resected pancreas adenocarcinoma showed a significant 1-year DFS of 63% and OS of 86%, which compares favorably with historical control subjects. As a result, a phase III trial of chemotherapy and chemoradiotherapy with or without algenpatnucel-L immunotherapy in 722 subjects with surgically resected pancreatic cancer has recently completed recruitment and results are eagerly awaited ( NCT01072981 ).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree