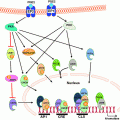
Fig. 3.1
Direct and indirect effects of adipokines, inflammatory mediators and insulin and IGF-1 on tumor cell growth. In obesity, hyperinsulinemia and high levels of certain adipokines, IGF-1 and inflammatory mediators can act directly on tumor cells to stimulate proliferation. These factors also stimulate estrogen production from the adipose which in turn further stimulate tumor cell proliferation
Adiponectin is produced by healthy mature adipocytes and its levels are inversely associated with obesity [55]. Many reports have suggested that adiponectin is protective against breast cancer development and progression [reviewed in 56]. In a study by Gulcelik et al., serum adiponectin levels were evaluated in 87 breast cancer patients and compared to cancer-free women [57]. Circulating adiponectin levels were found to be significantly lower in affected individuals compared to healthy controls (8,583 ± 2,095 ng/ml vs. 13,905 ± 3,263). The anti-cancer effects of adiponectin are hypothesized to be mainly due to the effect of adiponectin to reduce inflammation and increase insulin sensitivity [58]. However, effects of adiponectin on hormone biosynthesis and direct effects of adiponectin on cancer growth have also been described.
Conversely, leptin levels are higher in obese individuals, and higher leptin levels are significantly associated with an increase in breast cancer risk [59, 60]. Moreover, a prospective observational study by Macciò et al. demonstrated that leptin was also an independent predictor of tumor classification and TNM stage in postmenopausal women [61]. Leptin receptor expression is common in breast cancer [62], suggesting that leptin can act directly on tumor cells to modulate tumor growth. Leptin has been shown to activate a number of mitogenic pathways in cancer cells, including phosphoinositide 3-kinase/protein kinase B (PI3 K/AKT), mitogen-activated protein kinase (MAPK), mammalian target of rapamycin (mTOR) and signal transducer and activator of transcription 3 (STAT3) [63, 64]. In vitro, leptin has been shown to increase heat shock protein 70 (HSP70) expression in MCF-7 breast cancer cells [65], which in turn is known to increase cell proliferation [66]. In mouse models, leptin was shown to be responsible for the increase in expression of cyclin D1, a cell-cycle control protein necessary for mammary gland development, in MMTV-Wnt1 transgenic mice [67].
A less well characterized adipokine, nicotinamide phosphoribosyl-transferase (Nampt), also known as visfatin, has also been implicated in cancer [reviewed in 68]. This pleiotropic hormone has been shown to be secreted from visceral adipose depots and acts to regulate a number of metabolic processes including NAD biosynthesis. It has been shown to promote cell proliferation, inflammation and angiogenesis, and inhibit apoptosis. Nampt has also been shown to be secreted from tumor cells where it can act in an autocrine manner. Similarly, resistin, involved in stimulating low density lipoprotein production from the liver, has been shown to be positively associated with tumor size and stage, as well as ER status [69].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree