Adenosis and Sclerosing Lesions
The term adenosis refers to a group of benign breast lesions that have in common a pathologic increase in the number of mammary glandular units. Some forms of adenosis are characterized by an increase in the number of lobular acini without distortion of the lobular architecture (“simple adenosis”). In other types, there is an accompanying stromal proliferation that compresses and distorts the glands (e.g., sclerosing adenosis). Still other forms of adenosis are characterized by a haphazard, infiltrative proliferation of glands, with little or no distortion (e.g., microglandular adenosis [MGA], tubular adenosis, and secretory adenosis).
The term sclerosing lesions describes a group of proliferative breast lesions in which benign glands are entrapped and distorted by fibrous or fibroelastotic connective tissue. Included in this group are radial scars and complex sclerosing lesions.
The importance of recognizing the various patterns of adenosis and sclerosing lesions, and the reason they are considered together in this chapter, is that they may be mistaken for invasive carcinoma, particularly low-grade forms, such as tubular carcinoma and low-grade invasive ductal carcinoma.
SCLEROSING ADENOSIS
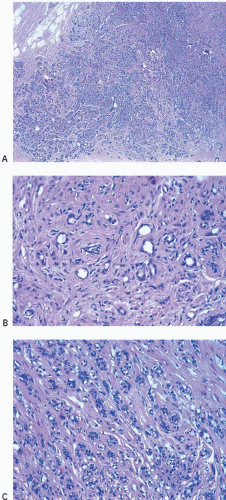
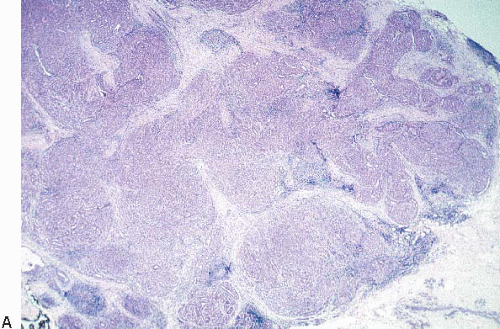
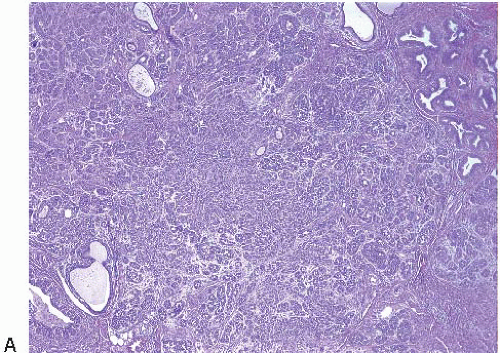
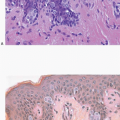
The most common form of adenosis is sclerosing adenosis, a lesion of the terminal duct lobular units characterized by a lobulocentric proliferation of glands and tubules accompanied by a stromal proliferation that produces variable glandular compression and distortion (Fig. 7.1, e-Fig. 7.1).
Sclerosing adenosis is usually an incidental microscopic finding; however, in some instances, it may present as a mammographic abnormality (most often microcalcifications). Less commonly, the lesion presents as a mammographic density or a palpable abnormality.
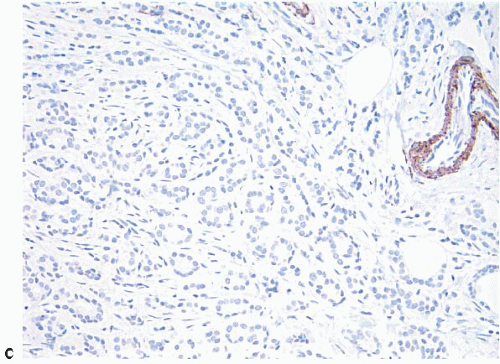
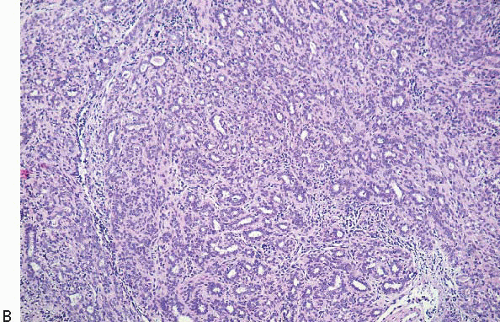
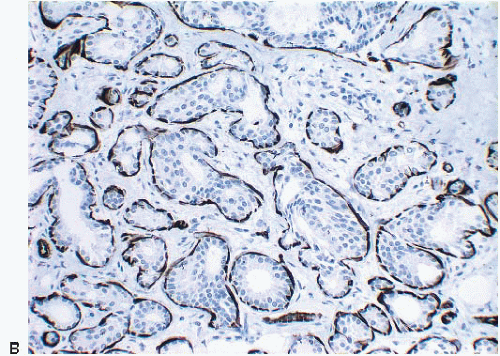
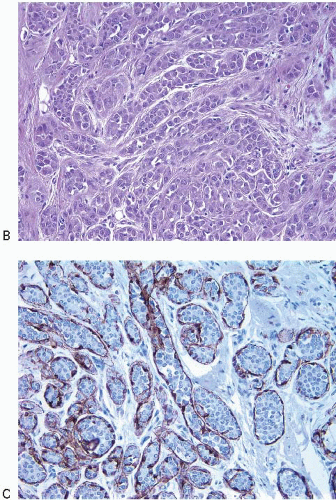
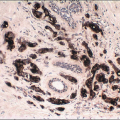
Lesions of sclerosing adenosis may be single or multiple. The glands and tubules comprising the proliferation are composed of benign, often flattened epithelial cells surrounded by a myoepithelial cell layer. Secretions may be present in gland lumina. The epithelial cells lack cytologic atypia; however, a worrisome picture may be seen when there is coexistent apocrine metaplasia (see subsequent text).1,2 The myoepithelial cell component in some cases is conspicuous, but in others it is difficult to appreciate on hematoxylin and eosin-stained sections. Even when inconspicuous on routinely stained sections, a myoepithelial cell layer around the glands is readily demonstrated by immunostains for myoepithelial cell markers such as calponin, smooth muscle myosin heavy chain, and p63 (Fig. 7.2).
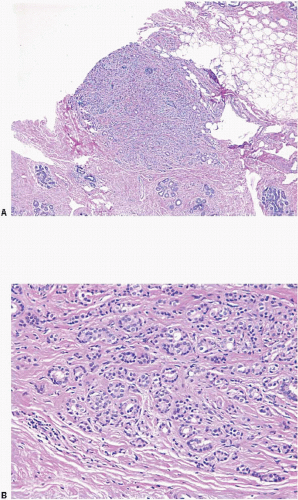
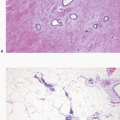
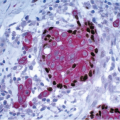
Glandular compression and distortion is most marked at the center of the lesion and may completely obliterate the lumina, resulting in the appearance of solid cords of cells within a fibrous stroma, often with a swirling pattern. Calcifications are frequently present in association with the glands (Fig. 7.3, e-Fig. 7.2). Perineural invasion, although uncommon, is a well-documented feature of sclerosing adenosis and should not be taken as an indication of malignancy (Fig. 7.4).3 The key to the diagnosis of sclerosing adenosis is low-power microscopic examination that demonstrates the circumscribed, lobulocentric nature of the process. This feature helps distinguish sclerosing adenosis from invasive carcinoma (Fig. 7.1A). However, infrequently, invasive carcinomas form relatively circumscribed foci, which at low power mimic the lobulocentric configuration of sclerosing adenosis (Fig. 7.5).
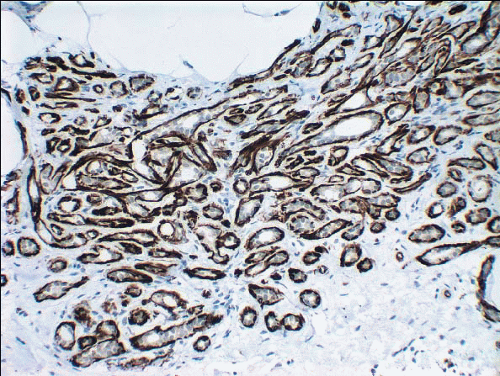 FIGURE 7.2 Sclerosing adenosis immunostained for smooth muscle myosin heavy chain highlighting the myoepithelial cells around the glands. |
The terms nodular adenosis and adenosis tumor have been applied to florid examples of sclerosing adenosis that results in a mammographic mass or palpable lesion. This lesion may sometimes be seen as rounded, pink, granular areas on gross examination. Microscopically, nodular adenosis is composed of aggregated or coalescent foci of typical sclerosing adenosis (Fig. 7.6, e-Fig. 7.3).
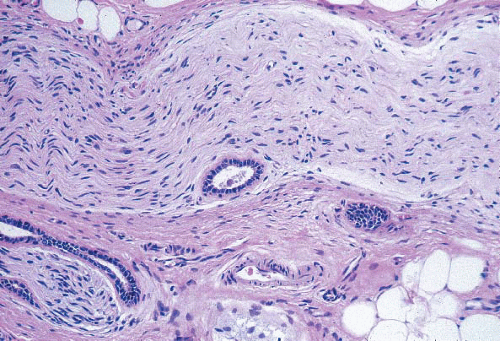 FIGURE 7.4 Perineural invasion in sclerosing adenosis. Benign glands are present adjacent to nerve twigs. |
The epithelium of the glands of sclerosing adenosis may demonstrate various proliferative lesions, including atypical hyperplasia and in situ carcinoma (either ductal or lobular types) (Figs. 7.7 and 7.8, e-Figs. 7.4 and 7.5). Involvement by in situ carcinoma produces an appearance of small nests, glands, or cords of neoplastic epithelial cells within a fibrotic stroma and the distinction from invasive carcinoma may be quite difficult; the use of myoepithelial cell immunostains may be required to confirm the in situ nature of the process (Figs. 7.7 and 7.8). However, even when involved by in situ carcinoma, the underlying lobulocentric configuration of sclerosing adenosis is retained, and it is important to recognize this in order to arrive at the correct diagnosis.
Clinical follow-up studies have indicated that sclerosing adenosis is associated with a 1.5- to 2-fold increase in the risk of the development of subsequent breast cancer, which is similar to the level of risk associated with other proliferative lesions without atypia.4 The presence of sclerosing adenosis in a core-needle biopsy specimen does not require surgical excision.
The key features of sclerosing adenosis are summarized in Table 7.1.
TABLE 7.1 Key Features of Sclerosing Adenosis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
APOCRINE ADENOSIS AND ATYPICAL APOCRINE ADENOSIS
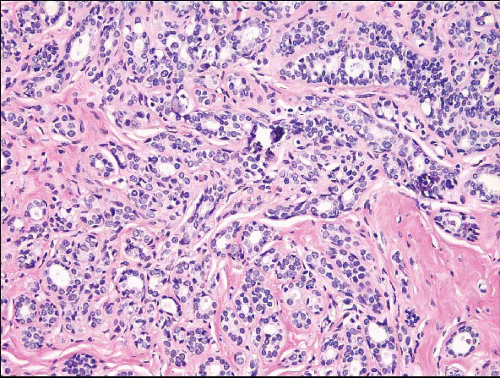
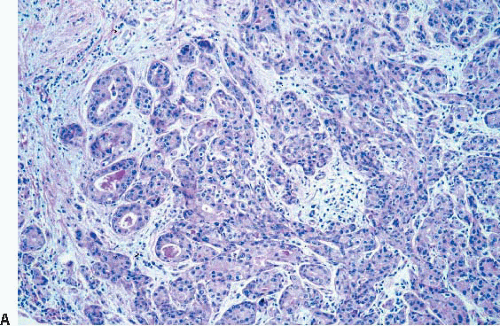
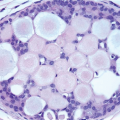
The term apocrine adenosis has been used to describe a variety of benign breast lesions in which the epithelium exhibits apocrine cytology.5,6 We and others have restricted this term to lesions that have the architectural features of sclerosing adenosis but in which the epithelium shows apocrine metaplasia as characterized by enlarged cell size, abundant granular, eosinophilic cytoplasm, large round nuclei, and prominent nucleoli (Fig. 7.9, e-Fig. 7.6).2 The nuclear enlargement and nucleolar prominence can create the erroneous impression of cytologic atypia unless the apocrine nature of the process is recognized.
The term atypical apocrine adenosis is applied when the apocrine cells show at least a threefold variation in nuclear size and nucleolar enlargement (Fig. 7.10).2 However, the distinction from apocrine ductal carcinoma in situ (DCIS) involving sclerosing adenosis is not clear-cut; this is especially problematic in cases in which there is apocrine DCIS elsewhere in the specimen.2,6 In equivocal cases, a diagnosis of “atypical apocrine adenosis” or “atypical apocrine proliferation involving sclerosing adenosis” is preferable. If the lesion is at or near a margin of an excision specimen, a re-excision may be prudent to exclude the possibility of adjacent diagnostic areas of apocrine DCIS. Finding such a lesion on a core-needle biopsy is an indication for excision, although the frequency with which a more advanced lesion is found on excision is not currently known.
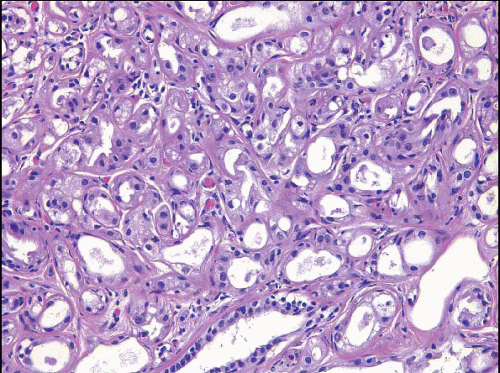 FIGURE 7.9 Apocrine adenosis. In this example of sclerosing adenosis, the epithelial cells have granular, eosinophilic cytoplasm and uniform round nuclei characteristic of apocrine metaplasia. |
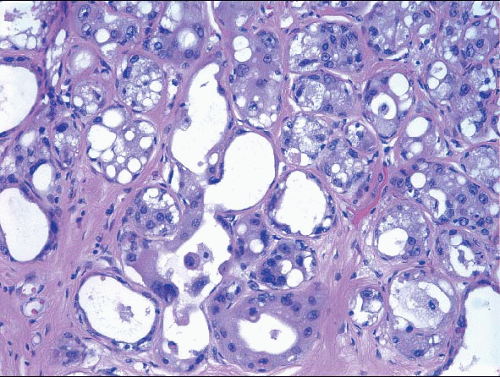 FIGURE 7.10 Atypical apocrine adenosis. The epithelial cells have eosinophilic cytoplasm typical of apocrine metaplasia, but show nuclear pleomorphism with prominent, enlarged nucleoli. |
It should be evident that when apocrine epithelium is present in sclerosing adenosis, the combination of epithelial cells with enlarged nuclei that have prominent nucleoli and glandular distortion may produce a pattern mimicking invasive carcinoma. This is particularly true for atypical apocrine adenosis. In such cases, immunostains for myoepithelial cell markers may be required to make this distinction.
The subsequent breast cancer risk associated with apocrine adenosis and atypical apocrine adenosis has not been well-defined. In one study, none of 47 patients with atypical apocrine adenosis developed cancer with a mean follow-up of 35 months.1 In contrast, in two other studies of 37 patients each with mean follow-up periods of almost 9 and 14 years, respectively, 3 patients developed carcinoma in one study and 4 in the other.2,7
MICROGLANDULAR ADENOSIS
MGA is an uncommon form of adenosis characterized by an infiltrative, nonlobulocentric proliferation of relatively uniform, small glands within the mammary stroma and adipose tissue (Fig. 7.11, e-Figs. 7.7 and 7.8).8, 9




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree