Key Points
- 1.
Adenocarcinoma of the endometrium is the most common gynecologic malignancy and is increasing in incidence. The mortality rate form this disease is also increasing.
- 2.
Classically, endometrial cancer is divided into two histologic types: type I is endometrioid, and type II is consists of uterine papillary serous carcinoma (UPSC), clear cell (CC), and other more aggressive histologic types.
- 3.
The mainstay of treatment is surgery with removal of the uterus tubes and ovaries with staging.
- 4.
Adjuvant therapy is often given. Radiation or chemotherapy is used depending on the clinical scenario.
- 5.
Endometrial cancer is commonly inherited through a DNA mismatch repair defect, which is known as Lynch syndrome. All patients with endometrial cancer should be evaluated for this possibility.
Incidence
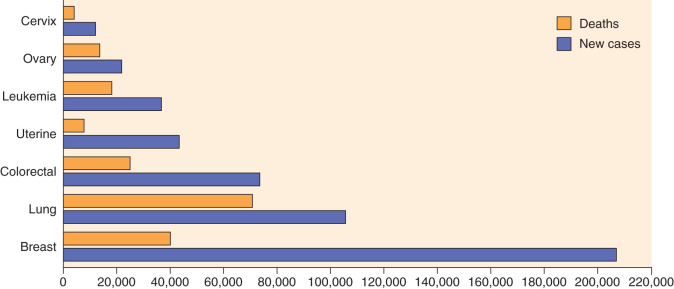
In the United States, cancer of the uterine corpus is the most common malignancy unique to women. It was estimated by the American Cancer Society that uterine cancer will develop in approximately 60,050 women in 2016 in the United States, making it the fourth most common cancer in women. The increased incidence of carcinoma of the endometrium has been apparent only during the past 3 decades. In reviewing the predicted incidence for the 1970s, the American Cancer Society noted a 1.5-fold increase in the number of patients with endometrial cancer; however, there was a decline in incidence during the late 1980s. In the past several years, the incidence has remained constant. During the period of increased incidence, predicted deaths from this malignant neoplasm actually decreased slightly. More recently, deaths from uterine cancer have increased. In 1990, the American Cancer Society estimated 4000 deaths from this cancer, increasing to 10,470 in 2016. An estimation of the most common new cancers and the percentage of female deaths for 2009 in the United States is shown in Fig. 5.1 . The increased use of estrogen has been implicated in the apparent increased incidence during the 1970s and early 1980s; however, Norway and Czechoslovakia report a 50% to 60% increase, respectively, in endometrial cancer even though estrogens are rarely prescribed or are not generally available there. However, the increasing prevalence of overweight and obesity in women, especially in developed countries, may account for this apparent increase. Regardless of the reason for the increased number of women with corpus cancer, this malignant neoplasm has become an important factor in the care of the female patient.
Epidemiology
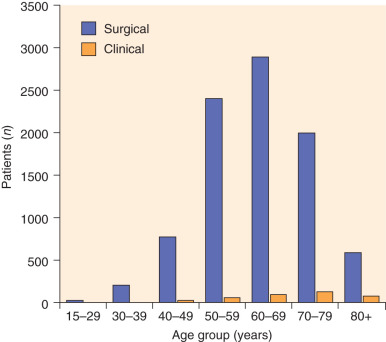
Endometrial adenocarcinoma occurs during the reproductive and menopausal years. The mean age for patients with adenocarcinoma of the uterine corpus is 63 years; most patients are between the ages of 50 and 59 years ( Fig. 5.2 ). Approximately 5% of women will have adenocarcinoma before the age of 40 years, and 20% to 25% will be diagnosed before menopause. Bokhman suggested that there are two pathogenic types of endometrial cancer. The first type arises in women with obesity, hyperlipidemia, and signs of hyperestrogenism, such as anovulatory uterine bleeding, infertility, late onset of menopause, and hyperplasia of the stroma of the ovaries and endometrium. The second pathogenic type of disease arises in women who have none of these disease states or in whom the disease states are not clearly defined. Bokhman’s data suggest that patients with the first pathogenic type mainly have well-differentiated or moderately differentiated tumors, superficial invasion of the myometrium, high sensitivity to progestins, and a favorable prognosis (85% 5-year survival rate in his material). The patients who fall into the second pathogenic group tend to have poorly differentiated tumors, deep myometrial invasion, a high frequency of metastatic disease in the lymph nodes, decreased sensitivity to progestin, and poor prognosis (58% 5-year survival rate).
Multiple risk factors for endometrial cancer have been identified, and MacMahon divides them into three categories:
- •
Variants of normal anatomy or physiology
- •
Frank abnormality or disease
- •
Exposure to external carcinogens
Obesity, nulliparity, and late menopause are variants of normal anatomy or physiology classically associated with endometrial carcinoma. These three factors are evaluated in regard to the possible risk of developing endometrial cancer in Table 5.1 . If a patient is nulliparous and obese and reaches menopause at age 52 years or older, she appears to have a fivefold increase in the risk of endometrial cancer above that of a patient who does not satisfy these criteria ( Table 5.2 ).
| Risk Factors | Risk |
|---|---|
| Obesity | 2.5–4.5× |
| Nulliparity | |
| Compared with one child | 2× |
| Compared with five or more children | 3× |
| Late menopause | 2.4× |
| Risk | ||
|---|---|---|
| Nulliparous | Parous | |
| Top 15% in weight | 5× more than | Lower two thirds in weight |
| Menopause at 52 years | Menopause before 49 years | |
The type of obesity in patients with endometrial cancer has been evaluated. In a study from the University of South Florida, it was noted that women with endometrial cancer had greater waist-to-hip circumference ratios, abdomen-to-thigh skin ratios, and suprailiac-to-thigh skin ratios than those of matched control women. As these ratios increased, the relative risk (RR) of endometrial cancer increased. The researchers concluded that upper-body fat localization is a significant risk factor for endometrial cancer. In a large multicenter case-control study of 403 endometrial cancer cases and 297 control cases, Swanson and associates confirmed and amplified these findings. Women whose weight exceeded 78 kg had a risk 2.3 times that of women weighing less than 58 kg. For women weighing more than 96 kg, the RR increased to 4.3. Upper-body obesity (waist-to-height ratio) was a risk factor independent of body weight. Patients in the highest quartile of both weight and waist-to-thigh circumference had a risk of 5.8 times. The amount of body fat has been associated with decreased circulating levels of both progesterone and sex hormone–binding proteins. There was a strong inverse association between sitting height and risk of endometrial cancer. This may be related to sex hormone–bound globulin (SHBG), which appears to be depressed in women with endometrial cancer. The level of SHBG is progressively depressed with increasing upper-body fat localization. With lower SHBG, there is a higher endogenous production of non–protein-bound estradiol. Because endometrial cancer is related to obesity, dietary habits appear to be important. Data suggest that the levels of estriol, total estrogens, and prolactin were lower and those of SHBG were higher in postmenopausal women who were vegetarians. In a case-control study, Levi and colleagues evaluated dietary factors in 274 patients with endometrial cancer and 572 control subjects from two areas in Switzerland and northern Italy. Extensive dietary history was obtained. Their data confirmed the relationship between obesity and endometrial cancer. In relation to diet, they noted an increased association with total energy intake. After correction for total energy intake, a risk was present with the frequency of consumption of most types of meats, eggs, beans, added fats, and sugar. Conversely, significant protection was noted with an elevated intake of most vegetables, fresh fruits, whole grain bread, and pasta. This reflected a low risk with increased intake of ascorbic acid and beta-carotene. Of dietary interest is that the intake of olive oil seemed beneficial in Switzerland but resembled other added fats in the Italian women. It has been previously noted that the amount and type of dietary fat influence estrogen metabolism because estrogen reabsorption from the bowel seems to be increased by diets rich in beef or fats.
Diabetes mellitus and hypertension are frequently associated with endometrial cancer. Elwood and colleagues reported an RR of 2.8 associated with a history of diabetes after controlling for age, body weight, and socioeconomic status. High levels of insulin-like growth factor I, coupled with elevated estrogen levels, are thought to have neoplastic potential that accounts for the observed increased risk of endometrial cancer. High blood pressure is prevalent in older obese patients but does not appear to be a significant factor by itself, even though 25% of patients with endometrial cancer have hypertension or arteriosclerotic heart disease.
As extensively detailed in Chapter 4 , the relationship of unopposed estrogen and endometrial cancer is well documented. Fortunately, the addition of a progestin appears to be protective. The adequacy of progesterone is important in prevention of endometrial cancer. In a study from Sweden, at the end of 5 years, excess risk of endometrial cancer was 6.6, but with combined estrogen progestin (E + P), the RR was 1.6 for 11 to 15 days of progestin, 2.9 for 10 days of use, and 0.2 if continuous E + P was given. The Million Women study from the United Kingdom reported its findings of endometrial cancer and hormone replacement therapy (HRT). This study, which first reported on HRT and breast cancer, has been severely criticized mainly on methodology factors. The study had an average follow-up of 3.4 years, during which 1320 incident endometrial cancers were diagnosed. At time of recruitment, 22% of HRT users (total number was 320,953 women) last used continuous combined therapy, 45% last used cyclic combined therapy with progestogen usually added for 10 to 14 days per month, 19% last used tibolone, and 4% used estrogen alone. Compared with nonusers, the RRs of endometrial cancer were 0.71, confidence interval (CI) 0.56 to 0.90, P = 0.005; 1.05, CI 0.91 to 1.22; 1.79, CI 1.43 to 2.25, P <0.001; and 1.45, CI 1.02 to 2.06, P = 0.04, respectively. Of note, the adverse effects of tibolone and estrogen only were greatest in the nonobese women, and the beneficial effects of combined HRT were greatest in obese women. Although the risk of unopposed estrogen is present, women taking estrogen who develop endometrial cancer appear to have favorable prognostic factors. Several but not all studies suggest that risk factors such as multiparity and obesity are lower in the estrogen users. Stage of disease and histologic grade appear to be lower in estrogen users. With correction for stage and grade, estrogen users still have less myometrial invasion than nonestrogen users do. The poor prognostic subtypes, such as clear cell carcinoma and adenosquamous cancer, appear less frequently in estrogen users. As a result, survival rates with estrogen-related endometrial cancer are much better than those of non–estrogen-related cancers. In fact, some studies note just as good if not better survival in estrogen users than in women with nonestrogen, nonendometrial cancers.
Data indicate that the use of combination oral contraceptives decreases the risk for development of endometrial cancer. The Centers for Disease Control and Prevention evaluated endometrial cancer cases of all women aged 20 to 54 years from eight population-based cancer registries and compared them with control patients selected at random from the same centers. A comparison of the first 187 cases with 1320 control cases showed that women who used oral contraceptives at some time had a 0.5 RR of developing endometrial cancer compared with women who had never used oral contraceptives. This protection occurred in women who used oral contraceptives for at least 12 months, and protection continued for at least 10 years after oral contraceptive use. Protection was most notable for nulliparous women. These investigators estimated that about 2000 cases of endometrial cancer are prevented each year in the United States by past or current use of oral contraceptives. Of interest, cigarette smoking appears to decrease the risk for developing endometrial cancer. In a population-based case-control study of women aged 40 to 60 years, Lawrence and associates found a significant decline in RR of endometrial carcinoma with increased smoking ( P >0.05). The RR decreased by about 30% when one pack of cigarettes was smoked per day and by another 30% when more than one pack was smoked per day. The effects of smoking did not appear to vary with menstrual status or exogenous estrogen. There was a fourfold increase in smoking-related odds ratio with body weight; the greatest reduction in risk by smoking was in the heaviest women. However, the estimated risk increased 12-fold in overweight women who were nonsmokers and whose primary source of estrogen was peripheral conversion of androgen to estrogen. Although smoking apparently reduces the risk for development of early-stage endometrial cancer, this advantage is strongly outweighed by the increased risk of lung cancer and other major health hazards associated with cigarette smoking.
Incidence and survival are higher in white women compared with black women. Reasons for these differences are unexplained. An analysis of the Gynecologic Oncology Group (GOG) database evaluated this factor in 600 white and 91 black women with clinical stage I or stage II endometrial cancer. A larger number of the African American women were diagnosed after age 70 years, and they had a higher proportion of UPSC and clear cell histologic types; the black women also had more advanced disease, grade, vascular space involvement, depth of invasion, and lymph node metastases than the white women did. The survival rate (5-year) was 77% for white women and 60% for black women. Survival difference remained even in high-risk groups such as grade III tumors (59% vs. 37%, respectively). The unadjusted hazard rate was 2.0, which was statistically significant. With adjustment for age, cell type, and extent of disease, the RR dropped to 1.2. The adjusted risk rate suggests that race is not a significant factor; nevertheless, race does denote an increased risk for poor prognostic factors, which clinically may be important.
Tamoxifen is used to prevent or treat breast cancer. Tamoxifen was first introduced in clinical trials in the early 1970s and was approved in 1978 by the US Food and Drug Administration (FDA) for treatment of advanced breast carcinoma in postmenopausal woman. Tamoxifen, although labeled an antiestrogen, is known to have estrogenic properties and truly is a weak estrogen. Women receiving tamoxifen also appear to have some protection from osteoporosis and heart disease (decreased lactate dehydrogenase and cholesterol), similar to women receiving estrogen replacement therapy. Extensive experience with this drug has been reported. It is estimated that more than 4 million women in the United States have taken tamoxifen for almost 8 million women-years of use. One of its major benefits is that in women taking tamoxifen, there has been a substantial decrease in the incidence of a second cancer in the opposite breast compared with similar women who were taking a placebo.
The Early Breast Cancer Trialists Collaborative Group (EBCTCG) has produced an important meta-analysis of 194 randomized trials of adjuvant chemotherapy or endocrine therapy with at least 15 years of follow-up. The analysis evaluated the effects of adjuvant tamoxifen on breast cancer recurrence and survival. It was shown that 5 years of tamoxifen therapy, compared with no adjuvant therapy, reduced the 15-year probability of breast cancer recurrence (from 45% to 33%) and breast cancer mortality (from 35% to 26%). In addition, tamoxifen has been shown to provide a preventive benefit in women at risk for developing breast cancer.
There has been a considerable amount of discussion in the literature concerning the association of tamoxifen with endometrial cancer. At least three studies (Fisher and colleagues, Powels and colleagues, and Veronesi and colleagues) evaluating the prophylactic use of tamoxifen in women without breast cancer have reported an association between tamoxifen use and endometrial cancer. In addition, several cases of endometrial cancer have been described in women receiving tamoxifen. In a prospective, randomized study of the National Surgical Adjuvant Breast and Bowel Project (NSABP), 2843 patients with node-negative estrogen receptor–positive invasive breast cancer were randomly assigned to receive a placebo or 20 mg/day of tamoxifen. An additional 1220 tamoxifen-treated patients were registered and given the drug. The average time in the study was 8 years for the randomly assigned patients and 5 years for the registered patients. Of the 1419 patients randomly assigned to tamoxifen, 15 developed uterine cancer, of which two were sarcomas. One patient randomly assigned to receive tamoxifen did not take the drug and developed endometrial cancer 78 months after randomization. In the placebo group, two developed endometrial cancer; however, both were receiving tamoxifen at the time of their uterine malignant disease. One patient had a breast recurrence and was prescribed tamoxifen, and the other was given tamoxifen after colon cancer. Two of the patients with endometrial cancer had been taking tamoxifen for only 5 and 8 months before their diagnosis of uterine disease was made. Five patients in the tamoxifen group developed endometrial cancer after the drug had been discontinued for 7 to 73 months. In the registered patients who received tamoxifen, eight uterine tumors (seven endometrial) were subsequently diagnosed. Three of these patients had been taking tamoxifen for less than 1 year (2 months, 2 months, and 9 months). The authors determined the average annual hazards ratio (HR) of endometrial cancer per 1000 women in their population of patients. This was 0.2 per 1000 in the placebo group and 1.6 per 1000 for the randomized tamoxifen-treated patients. In the registered patients receiving tamoxifen, the average annual hazard rate was 1.4 per 1000, similar to that of the randomized tamoxifen-treated group. The hazard rate of endometrial cancer in the placebo group was low compared with the Surveillance, Epidemiology, and End Results (SEER) data and with previous NSABP randomized tamoxifen–placebo studies; these data suggest that the average annual hazard rate is 0.7 per 1000.
These data, based on a limited number of patients with endometrial cancer while receiving tamoxifen, suggest that there may be an RR of 2.3 for development of endometrial cancer while receiving tamoxifen. This does not take into account the well-known fact that women who develop breast cancer are at an increased risk for development of endometrial cancer irrespective of subsequent treatment. The RR of 1.72 to more than 3 has been reported. The risks and benefits of the prevention of recurrences and new breast cancer in comparison to new endometrial cancers were evaluated in the NSABP study. The benefits suggest that 121 fewer breast-related events per 1000 women treated with tamoxifen were seen compared with 6.3 endometrial cancers per 1000 women. Therefore, the benefit from tamoxifen is apparent.
It was initially suggested that the rate of endometrial cancers associated with tamoxifen use might be equal to that associated with unopposed estrogen replacement therapy. Because tamoxifen is a weak estrogen, similar characteristics of endometrial cancer were also implied (ie, well-differentiated superficially invasive cancers). Barakat and associates reviewed five studies, including the study by Magriples, the NSABP, their own data from Memorial Sloan-Kettering Hospital, and two studies from overseas. A total of 103 patients were evaluated in regard to histologic features, grade of tumor, International Federation of Gynecology and Obstetrics (FIGO) staging, and deaths from uterine cancer; an increase was not found in poor prognostic histologic findings, tumor differentiation, or stage compared with what would be expected in a similar group of non–tamoxifen-treated patients with uterine cancer. Jordan, in an evaluation of the SEER data and of tamoxifen-associated endometrial cancer in the literature, reported similar findings.
It is suggested that all women, irrespective of whether they are taking tamoxifen, should have yearly gynecologic examinations. The endometrium should be evaluated if the patient is symptomatic. We concur with the American College of Obstetricians and Gynecologists (ACOG) committee opinion that does not recommend endometrial sampling or ultrasound evaluation of the endometrium just because an individual is taking tamoxifen. This possible concern of tamoxifen and endometrial cancer may lessen in the near future because the aromatase inhibitors (AIs) may appear to be better than tamoxifen in the prevention of recurrent or contralateral breast cancer. Several clinical trials have demonstrated the comparable if not greater efficacy of AIs compared with tamoxifen. Although tamoxifen remains an option for adjuvant therapy for postmenopausal women, AIs are thought to be more effective in preventing breast cancer recurrence in the first 2 years after surgery. AIs reduce estrogen levels in postmenopausal women by inhibiting or inactivating aromatase, the enzyme that synthesizes estrogens from circulating androgens. AIs should be avoided in premenopausal women, including those who have experienced chemotherapy-induced amenorrhea. Whereas tamoxifen is a partial agonist, AIs are not agonists and are not associated with estrogenic-related thromboembolic events and uterine cancers. The activity of third-generation agents anastrozole, letrozole, and exemestane is generally considered comparable. Although AIs are associated with a significant risk of osteoporosis, they do not increase the risk of gynecologic problems. In one large adjuvant therapy trial (the Anastrozole, Tamoxifen Alone or in Combination Trial [ATAC]), anastrozole was associated with fewer cerebrovascular events (2.0% vs. 2.8%), endometrial cancers (0.2% vs. 0.8%), thromboembolism (3% vs. 4%), hot flashes (41% vs. 36%), and vaginal bleeding (5% vs. 10%) compared with tamoxifen. The role of AIs as prophylaxis is being investigated, but data are lacking at the present time.
Although most cases of endometrial cancer are sporadic, hereditary endometrial cancer has been identified in association with hereditary nonpolyposis colon cancer (HNPCC), also known as Lynch II syndrome. This is an autosomal-dominant inherited cancer that involves a germline mutation in one of the genes in the DNA mismatch repair gene family, which includes MSH2, MLM1, and MSH6 . Fortunately, HNPCC accounts for only 1% to 5% of all colorectal cancers but it is associated with a 39% to 54% lifetime risk of developing colon cancer. There is a lifetime risk of 30% to 61% of developing endometrial cancer in those with HNPCC. There is also an increased risk of ovarian cancers and other nongynecologic cancers. In a study by Lu and associates, they noted that about half the time, the endometrial or ovarian cancer appeared before the colon cancer. In both instances, the age at diagnosis was in the early 40s. There was a median of 11 years between the gynecologic cancer and the colon cancer. About 14% of the time, the gynecologic and colon cancers were diagnosed simultaneously. Several “red flags” should prompt an evaluation for HNPCC. These include any individual with a personal or family history of colon cancer at an early age of onset (usually before age 50 years) or endometrial cancer at an early age of onset (premenopausal or before age 50 years) and two or more HNPCC-related cancers in an individual or family. Assessing a patient’s risk for hereditary cancer is an important process, beginning with screening for the “red flags” of hereditary colon (and endometrial) cancer. Individuals with any of the “red flags” should enter into a discussion about genetic testing to determine if this is appropriate for them. Medical management strategies can be tailored depending on the genetic testing results and may include increased surveillance, chemoprevention, and prophylactic surgery. The Cancer Study Consortium suggests colonoscopy every 1 to 3 years beginning at age 25 years in individuals with this hereditary disorder. The data suggest that if surveillance is done, survival rates are improved. Women should be offered surveillance with ultrasound and endometrial sampling from age 25 to 35 years, although there are no data to suggest this will improve survival if endometrial cancer is diagnosed by these means. As reported by Schmeler and colleagues, risk-reducing surgery consisting of removal of the uterus and bilateral ovaries has been shown to decrease the risk of uterine and ovarian cancer in these high-risk patients.
Diagnosis
Routine screening for uterine adenocarcinoma and its precursors is not recommended. Women receiving HRT (estrogen and progesterone) do not need endometrial biopsy before institution of therapy or during replacement therapy unless abnormal bleeding occurs. Monthly withdrawal bleeding after progestin is not considered abnormal bleeding. However, breakthrough bleeding should be evaluated. The use of continuous estrogen alone increases the risk of adenocarcinoma. Estrogen plus progesterone appears to decrease the risk of adenocarcinoma and therefore is the preferred treatment. In asymptomatic high-risk patients, periodic screening may be advisable. All postmenopausal women with uterine bleeding must be evaluated for endometrial cancer, although only 20% of these patients will have a malignant genital neoplasm. As the patient’s age increases after menopause, there is a progressively increasing probability that her uterine bleeding is caused by endometrial cancer. Feldman and associates found that age was the greatest independent risk factor associated with endometrial cancer or complex hyperplasia. In women aged 70 years or older, the odds ratio was 9.1. If complex hyperplasia was present, the odds ratio increased to 16. When a woman was older than 70 years, her chance of having cancer when vaginal bleeding was present was about 50%. If she was also nulliparous and had diabetes, the risk was 87%. A perimenopausal patient who may have abnormal uterine bleeding indicative of endometrial cancer is frequently not evaluated because the patient or her physician interprets her new bleeding pattern as resulting from menopause. During this time in a woman’s life, the menstrual periods should become lighter and lighter and farther and farther apart. Any other bleeding pattern should be evaluated with carcinoma of the endometrium in mind. A high index of suspicion must be maintained if the diagnosis of endometrial cancer is to be made in a young patient. Prolonged and heavy menstrual periods and intermenstrual spotting may indicate cancer, and endometrial sampling is advised. Most young patients who develop endometrial cancer are obese, in many instances massively overweight, often with anovulatory menstrual cycles.
Historically, fractional dilation and curettage (D&C) has been the definitive diagnostic procedure used in ruling out endometrial cancer. Today, most advocate the routine use of the endometrial biopsy as an office procedure to make a definitive diagnosis and spare the patient hospitalization and an anesthetic. Several studies have indicated that the accuracy of the endometrial biopsy in detecting endometrial cancer is approximately 90%. Cytologic detection of endometrial cancer by routine cervical Papanicolaou (Pap) smear has generally been poor in comparison with the efficacy of the Pap smear in diagnosing early cervical neoplasia. Several studies in the literature indicate that only one-third to half of the patients with adenocarcinoma of the endometrium have abnormal Pap smear results on routine cervical screening. The main reason for the poor detection with the cervical Pap smear is that cells are not removed directly from the lesion. When a cytologic preparation is obtained directly from the endometrial cavity, malignant cells are present in higher numbers than those found if routine cervical or vaginal smears are obtained. Techniques that obtain only a cytologic preparation are generally inadequate if they are used alone.
Several commercial apparatuses are available for sampling the endometrial cavity on an outpatient basis. If diagnosis of endometrial cancer can be made on an outpatient basis, the patient can avoid hospitalization and a minor surgical procedure. Devices that remove tissue for histologic evaluation have generally been good if tissue is obtained from the endometrial cavity. Stovall and colleagues used the Pipelle instrument to evaluate 40 patients known to have endometrial cancer. Ninety percent of the women were postmenopausal. Only in one patient was cancer not identified with the Pipelle. This patient had a prior D&C that revealed a grade I lesion. The Pipelle diagnosis was atypical adenomatous hyperplasia, and the hysterectomy specimen revealed a focus of adenocarcinoma in situ. The pathologist noted that the obtainable tissue was acceptable for analysis in 100% of patients. Discomfort was recorded as mild in 80%, and only two patients (5%) reported severe pain. Goldchmit and coworkers reported similar accuracy with the Pipelle in 176 consecutive patients undergoing D&C. Whereas endometrial biopsy and D&C appear to be equivalent in terms of diagnosing cancer, the accuracy of endometrial biopsy appears to be inferior to D&C in predicting final posthysterectomy tumor grade. In a recent study by Leitao and colleagues, 18% of endometrial biopsy specimens were upgraded on final hysterectomy specimen, but only 9% of D&C specimens were upgraded. In symptomatic patients in whom inadequate tissue (or no tissue at all) is obtained for pathologic evaluation, a D&C must be considered.
Hysteroscopy has been suggested as adjuvant in making the diagnosis of endometrial cancer and in establishing the extent of disease. Hysteroscopy has been used frequently in the evaluation of patients with abnormal uterine bleeding and has the advantages of allowing the physician to see the pathologic lesion and direct biopsy, identify other competing diagnoses (fibroids, polyps), and perform the procedure on an outpatient basis. Clark and colleagues analyzed data from 65 primary studies on the use of hysteroscopy to diagnose endometrial cancer and endometrial disease (cancer, hyperplasia, or both), including more than 26,000 women. All of the patients had abnormal premenopausal or postmenopausal uterine bleeding. Using endometrial histologic findings as a reference, a positive hysteroscopy result was associated with a 72% probability of endometrial cancer, but a negative result reduced this probability to 0.6%. The corresponding probabilities for endometrial disease were 55% with a positive result and 3% with a negative result. The accuracy of hysteroscopy tended to be higher among postmenopausal women and in an outpatient setting. They concluded that hysteroscopy is highly accurate and thereby clinically useful in diagnosing endometrial cancer in women with abnormal uterine bleeding and is moderately useful in diagnosing endometrial disease. Because many patients with endometrial cancer can be diagnosed with office biopsy, that is our preferred first diagnostic step. If the biopsy result is negative and further evaluation is needed, we proceed to hysteroscopy. With its use, surgeons can direct biopsies of focal lesions that might be missed by D&C. Hysteroscopy can also be used to evaluate the endocervical canal.
Ultrasonography has been suggested as a diagnostic tool in evaluating women with irregular bleeding, particularly postmenopausal patients ( Fig. 5.3 ). The endometrial stripe as seen with transvaginal ultrasonography appears to be indicative of endometrial thickness. Several studies suggest that if a thin endometrial stripe is present, a histologic diagnosis is not necessary because atrophic endometrium would be present. Granberg and associates evaluated 205 women with postmenopausal bleeding, 30 postmenopausal asymptomatic women, and 30 postmenopausal patients with known endometrial cancer. In the two groups of 60 patients, the endometrial thickness was 3.2 (mean) versus 17.7, respectively. In the group of 205 women, 18 were found to have endometrial cancer. No cancers were present in the endometrium that had an endometrial thickness of 8 mm or less. There was considerable overlap of endometrial thickness by all histologic groups. The authors noted that if a cutoff of 5 mm was used, no false-negative findings were present. With this measurement, the positive predictive value was 87%, with specificity of 96% and sensitivity of 100% for identifying endometrial abnormalities. It has been suggested that if ultrasonography could save a large number of endometrial biopsies, there would be a large cost savings with less discomfort to the patient. As previously noted, significant pain with the newer disposable endometrial biopsy techniques affects only a small number of patients, and a certain number of patients, because of considerable endometrial thickness, will require endometrial sampling anyway. Clark and colleagues investigated the cost-effectiveness of initial diagnostic strategies for postmenopausal bleeding. A decision analytic model was constructed to reflect current service provision, which evaluated 12 diagnostic strategies using endometrial biopsy, ultrasonography (4- and 5-mm endometrial thickness cutoff), and hysteroscopy. Diagnostic probability estimates were derived from systematic quantitative reviews, clinical outcomes from published literature, and cost estimates from local and National Health Service sources. The main outcome measure was the cost per additional life year gained (£/LYG). Compared with carrying out no initial investigation, a strategy based on initial diagnosis with ultrasonography using a 5-mm cutoff was the least expensive (£11 470/LYG). Initial investigation with endometrial biopsy or ultrasonography using a 4-mm cutoff was comparably cost-effective (<£30,000/LYG vs. ultrasonography with a 5-mm cutoff). The strategies involving initial evaluation with test combinations or hysteroscopy alone were not cost-effective. They concluded that women presenting for the first time with postmenopausal bleeding should undergo initial evaluation with ultrasonography or endometrial biopsy.
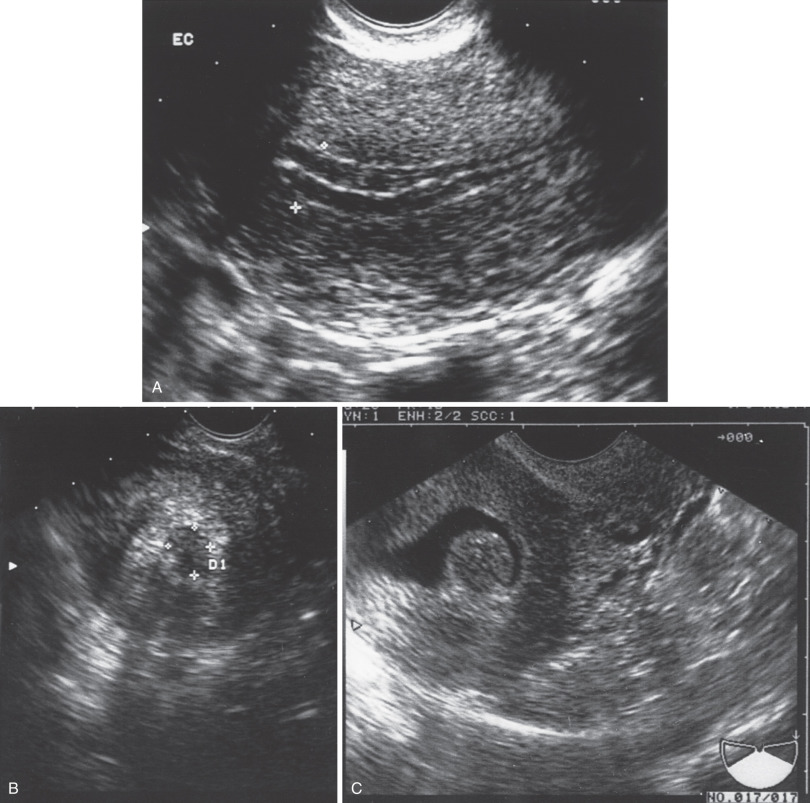
Unfortunately, endometrial cancer has been identified when the endometrial thickness is less than 5 mm. Although studies may evaluate several hundred patients, most do not have many cancer patients included. Wang and associates reviewed the ultrasounds of 52 women who were diagnosed with UPSC, clear cell and other high-grade carcinomas. Of the 52, 34 (65%) had a thickened endometrium measuring 5 mm or more; in nine (17%) the endometrium was less than 5 mm, and in an additional nine women (17%) the endometrium was indistinct. In the women with a nonthickened endometrium, other ultrasound abnormalities were noted: intracavitary fluid or lesion, myometrial mass, enlarged uterus, or adnexal mass. Multiple factors can affect endometrial thickness. These include estrogen use, E + P use, body mass index (BMI), diabetes, poor histotype, race, and postmenopausal status. The Committee on Gynecologic Practice of the ACOG issued an opinion on the role of transvaginal ultrasonography in the evaluation of postmenopausal bleeding. They concluded that women with postmenopausal bleeding may be assessed initially with either endometrial biopsy or transvaginal ultrasonography. This initial evaluation does not require performance of both tests. Transvaginal ultrasonography can be useful in the triage of patients in whom endometrial sampling was performed but tissue was insufficient for diagnosis. When transvaginal ultrasonography is performed for patients with postmenopausal bleeding and an endometrial thickness of less than or equal to 4 mm is found, endometrial sampling is not required. Meaningful assessment of the endometrium by ultrasonography is not possible in all patients. In such cases, alternative assessment should be completed. When bleeding persists despite negative initial evaluations, additional assessment is indicated.
The reliability determining endometrial thickness in postmenopausal patients does not appear to be applicable to women taking tamoxifen. In all studies, the endometrium in a tamoxifen-treated patient is considerably thicker than in a non–tamoxifen-treated patient. Histologic evaluation revealed an atrophic endometrium in a large number of these tamoxifen-treated patients. Lahti and colleagues evaluated 103 asymptomatic postmenopausal patients (51 receiving tamoxifen and 52 control participants) with ultrasonography, hysteroscopy, and endometrial histologic examination. In the tamoxifen group, 84% had an endometrial thickness on ultrasonography of 5 mm or more versus 19% in the non-tamoxifen group (51% vs. 8% >10 mm, respectively). Hysteroscopy findings noted that 28% of uterine mucosa was atrophic versus 87% in the non-tamoxifen control group. Histopathologic examination noted atrophic endometrium in 60% of tamoxifen-treated patients versus 79% of control subjects. The biggest difference between the two groups was the finding of polyps in 18% of the tamoxifen group versus 0% of the control group; this appears to be a frequent finding in the tamoxifen-treated patient. So-called megapolyps measuring up to 12 cm have been described. Other uterine diseases have been attributed to tamoxifen, including increased uterine volume, lower impedance to blood flow in uterine arteries, endometriosis, focal periglandular condensation of stromal cells, and epithelial metaplasia. Data now suggest that the markedly thickened endometrium (≤40 mm) in patients receiving tamoxifen is not thickened endometrium but proximal myometrium.
Ultrasonography has also been evaluated as a means for determining depth of myometrial invasion. Gordon and associates studied 15 known patients with endometrial cancer by ultrasonography and magnetic resonance imaging (MRI). By use of criteria of greater than 50% myometrial wall involvement as deep invasion and less than 50% as superficial invasion, ultrasonography was judged to be more accurate than MRI in five studies; MRI was better in three, both were equally accurate in four, and neither was accurate in three. It has been suggested by some that ultrasonography can accurately predict myometrial invasion in about 75% of cases. Although knowing the depth of invasion preoperatively would be important information to the clinician, the data from studies as noted before would currently appear to be too premature or too costly to use routinely. We prefer to evaluate depth of myometrial invasion intraoperatively with gross examination or frozen section.
Pathology
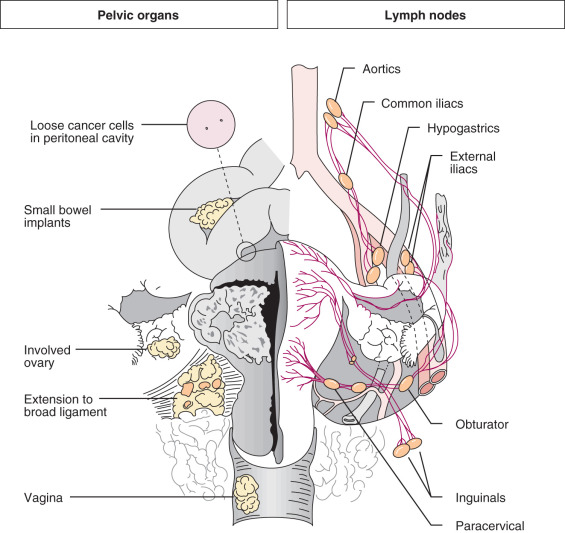
Careful evaluation of the uterus by the pathologist is essential for proper diagnosis and treatment of corpus cancer ( Fig. 5.4 ). Gross inspection of a bivalved uterus at the time of hysterectomy can offer an impression of the size of the lesion, its location (involvement of fundus, lower uterine segment, or cervix), and the depth of tumor penetration into the myometrium (depth of invasion). A clinically enlarged uterus may be caused by increasing tumor volume, but this should not be the only gauge for significant local disease. Obviously, many patients can have enlarged uteri because of factors other than adenocarcinoma. Carcinoma of the endometrium may start as a focal discrete lesion, as in an endometrial polyp. It may also be diffuse in several different areas, in some situations involving the entire endometrial surface. As the tumor grows, it can become larger or spread within the endometrium or myometrium. Endometrial cancer may disseminate to regional lymph nodes, by embolization or direct extension into the pelvis or vagina, or hematogenously to distant organs ( Fig. 5.5 ). The risk of spread is related to several factors, including depth of invasion into the myometrium, tumor grade, and histologic type.
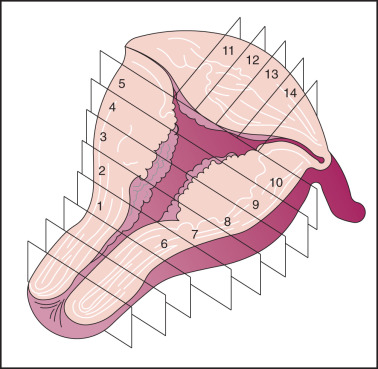
Adenocarcinoma, the most common histologic type, is usually preceded by a predisposing lesion, atypical endometrial hyperplasia ( Table 5.3 ). Only hyperplasias with cellular atypia are considered precursors of adenocarcinoma of the endometrium. For most patients with endometrioid-type tumors, particularly grade I or II lesions, and hyperplasia, hyperestrogenism is the etiologic basis. Pathologically, endometrial cancer is characterized by the presence of glands in an abnormal relationship to each other, with the hallmark of little, if any, intervening stroma between the glands. There can be variations in the size of the glands, and infolding is common. The cells are usually enlarged, as are the nuclei, along with nuclear chromatin clumping and nucleolar enlargement. Mitosis may be frequent. Differentiation of adenocarcinoma (mild, moderate, and severe, or grades I–III) is important prognostically and is incorporated into FIGO surgical staging ( Fig. 5.6 ). Most studies suggest that 60% to 65% of all endometrial cancers are of this subtype.
| Type | Number (%) |
|---|---|
| Endometrioid | 6231 (84) |
| Adenosquamous | 317 (4.2) |
| Mucinous | 74 (0.9) |
| Uterine papillary serous | 335 (4.5) |
| Clear cell | 185 (2.5) |
| Squamous cell | 28 (0.04) |
| Other | 285 (3.8) |
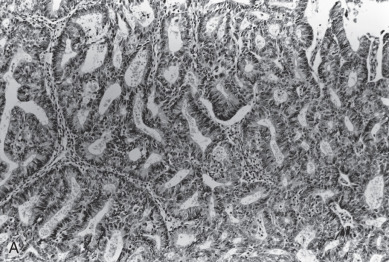
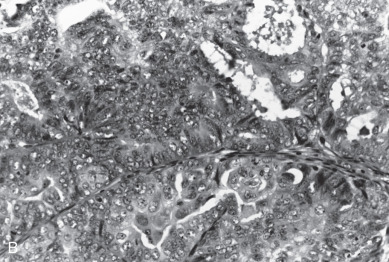
For almost a century, it has been recognized that a squamous component may be associated with an adenocarcinoma of the endometrium. This occurs in about 25% of patients. Historically, patients with a squamous component were further stratified according to whether the squamous component appeared benign (designated adenoacanthoma [AA]) or malignant (designated adenosquamous carcinoma [AS]). It was suggested that AA indicated a good prognosis and those with AS had a poor survival. Today, this distinction has been questioned concerning its prognostic importance. Zaino and coworkers, in reporting data from the GOG, suggest that the notation of squamous component irrespective of differentiation does not affect survival. Patients with clinical stage I and stage II cancers were evaluated, and 456 with typical adenocarcinoma (AC) and 175 with squamous differentiation (AC + SQ) were identified. The latter were subdivided into 99 with AA and 69 with AS. Multiple known prognostic factors were compared with differentiation of glandular and squamous component of the tumor. Age, depth of myometrial invasion, architecture, nuclear grade, and combined grade were similar for AC and AC + SQ, although patients with AA were better differentiated than those with AS and had less myometrial invasion. Both glandular and squamous differentiation correlated with frequency of pelvic and paraaortic node metastasis. Nodal metastasis, when it was stratified for grade and depth of invasion, was similar in AC and AC + SQ patients. The differentiation of squamous component is closely correlated with the differentiation of the glandular element, and the glandular element is a better predictor of outcome. It would therefore appear that the previous designation of AA and AS has no added predictive property than differentiation of glandular component and probably should be dropped as a diagnostic term. The authors suggest the term squamous differentiation instead, with differentiation of the glandular component noted as the important prognostic factor. Subsequently, Abeler and Kjorstad reviewed 255 cases and made the same recommendations.
Secretory adenocarcinoma ( Fig. 5.7 ) is an uncommon type of endometrial cancer. It usually represents well-differentiated carcinoma with progestational changes. It is difficult to differentiate it from secretory endometrium. Survival is good and comparable to that associated with the pure adenocarcinoma. Although it is an interesting histologic variant, the separation of the entity as it relates to treatment and survival is probably not warranted.
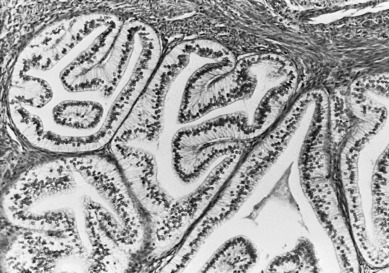
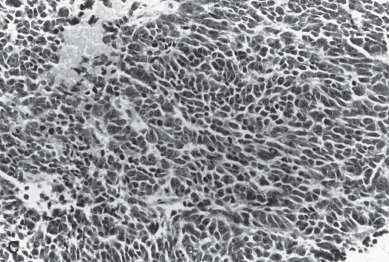
Increasing emphasis has been placed on the importance of the histologic subtype serous adenocarcinoma of the uterus, known as uterine papillary serous carcinoma (UPSC). This subtype represents fewer than 10% of all adenocarcinomas but is a highly aggressive carcinoma of the uterus. Unlike the more common variants, UPSC is not associated with hyperestrogenism and frequently develops in the setting of atrophic endometrium. It is more commonly seen in older and nonwhite patients. Hendrickson, in the early 1980s, noted that in more than 250 endometrial cancers, only 10% had histologic features of UPSC, but these accounted for 50% of all treatment failures. The histopathologic appearance resembles a high-grade serous carcinoma of the ovary that has a propensity for vascular or lymphatic vascular space involvement (LVSI). Well-formed papillae are lined by neoplastic cells with grade III cytologic features ( Fig. 5.8 ). Differentiation between papillary architecture and syncytial metaplasia with benign endometrial alterations must be made because the papillary architecture alone does not designate UPSC. The uterus may appear grossly normal but can have extensive myometrial invasion. Most UPSC tumors are aneuploid and have a high S-phase, and can be pure or admixed with other histologic types (endometrioid, clear cell, carcinosarcoma).
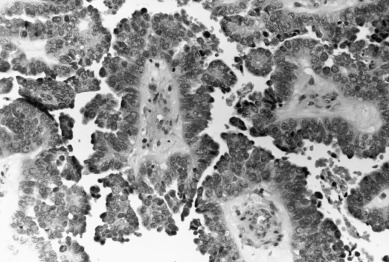
Clear cell carcinomas ( Fig. 5.9 ) are also infrequent in number but have distinct histologic criteria. Clear cell tumors are characterized by large polyhedral epithelial cells that may be admixed with typical non–clear cell adenocarcinomas. Some authorities accept the mesonephritic-type hobnail cells as part of this pattern, but others believe that this histologic type should be excluded from the clear cell category. Silverberg and DeGiorgi and Kurman and Scully suggested a worse prognosis for clear cell adenocarcinoma than for pure adenocarcinoma. This was confirmed in studies by Christopherson and coworkers. Even in stage I disease, only 44% of patients with clear cell carcinomas survive 5 years. Neither the FIGO classification nor nuclear grade correlates with survival. Photopulos and associates, in a review of their material, noted that their patients with this entity were older and tended to have a worse prognosis. They did note that patients with stage I clear cell carcinomas had a 5-year survival rate similar to that of patients with stage I pure adenocarcinoma of the endometrium.
Tumor Grade
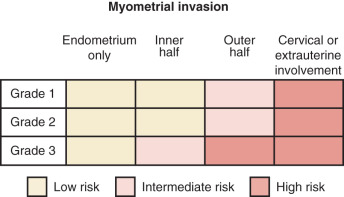
In addition to histologic type, pathologists assign a measure of tumor differentiation, known as grade, to endometrial cancers. Grade I lesions are well differentiated, are frequently associated with estrogen excess, closely resemble hyperplastic endometrium, and are generally associated with a favorable prognosis. Grade III lesions are poorly differentiated, do not resemble normal endometrium, and frequently have a poorer prognosis. Grade II tumors are moderately well differentiated and have an intermediate prognosis. Both architectural criteria and nuclear grade are used to classify. Architectural grade is related to the proportion of solid tumor growth, with grade I having an adenocarcinoma in which less than 5% of the tumor growth is in solid sheets, grade II having 6% to 50% of the neoplasm arranged in solid sheets of neoplastic cells, and grade III having greater than 50% of the neoplastic cells in solid masses. Regions of squamous differentiation are excluded from this assessment. The FIGO rules for grading state that notable nuclear atypia, inappropriate for architectural grade, raises the grade of a grade I or grade II tumor by one. By convention, serous and clear tumors are considered grade III or high-grade tumors.
Prognostic Factors
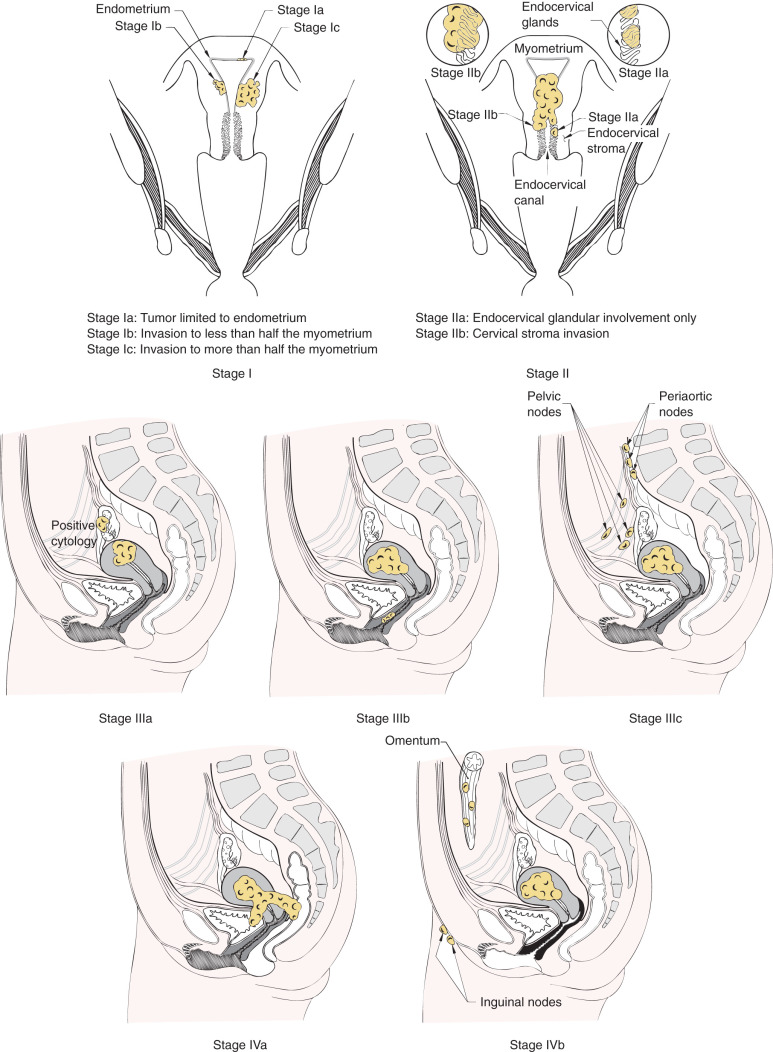
After hysterectomy and lymph node dissection, clinical-pathologic characteristics are commonly used to predict risk of recurrence and to optimize therapy. Multiple factors have been identified for endometrial carcinoma that has prognostic value ( Table 5.4 ). Essentially all reports in the literature agree stage (extent of disease spread), grade of tumor, and depth of invasion are important prognostic considerations. Before 1988, endometrial cancer was clinically staged with stage assignments based on uterine size and clinical extent of disease. Because of the considerable discrepancy between the clinical extent of disease spread and pathologic spread noted after surgical staging, FIGO adopted a surgical-pathologic staging classification in 1988. In 2009, FIGO updated the staging system ( Table 5.5 ). FIGO staging classification attempts to categorize patients into prognostic groups based on extent of disease and tumor grade.
| Histologic type (pathology) Histologic differentiation Stage of disease Myometrial invasion Peritoneal cytology Lymph node metastasis Adnexal metastasis |
| Stage I * | Tumor Contained to the Corpus Uteri | ||
| Ia | No or less than half myometrial invasion | ||
| Ib | Invasion equal to or more than half of the myometrium | ||
| Stage II | Tumor invades the cervical stroma but does not extend beyond the uterus † | ||
| Stage III * | Local and/or regional spread of tumor ‡ | ||
| IIIa | Tumor invades the serosa of the corpus uteri and/or adnexa | ||
| IIIb | Vaginal and/or parametrial involvement | ||
| IIIc | Metastases to pelvis and/or paraaortic lymph nodes | ||
| IIICl | Positive pelvic nodes | ||
| IIIC2 | Positive paraaortic lymph nodes with or without positive pelvic lymph nodes | ||
| Stage IV * | Tumor invades bladder and/or bowel mucosa and/or distant metastases | ||
| IVa | Tumor invasion of bladder and/or bowel mucosa | ||
| IVb | Distant metastases, including intraabdominal metastases and/or inguinal lymph nodes | ||
† Endocervical glandular involvement only should be considered as stage I and no longer as stage II.
‡ Positive cytology has to be reported separately without changing the stage.
Stage of Disease: Depth of Invasion, Cervical Involvement, Adnexal Involvement, and Nodal Metastasis
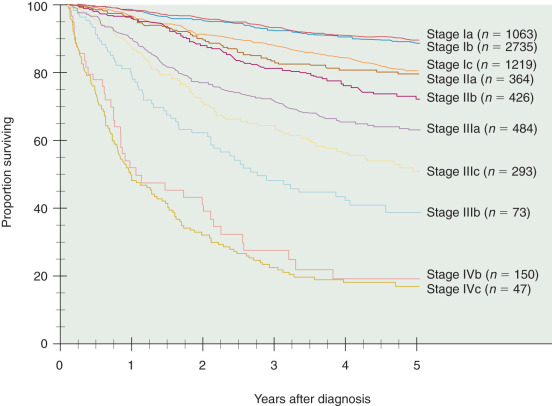
Staging of patients with malignancies is designed to have prognostic value by classifying the size and extent of tumor. The survival rate in regard to stage of disease has been consistent, and Table 5.6 and Fig. 5.10 show the 5-year survival rate reported by FIGO (FIGO 1988 staging). The patterns of disease spread in endometrial cancer was evaluated in a prospective study performed by the GOG (GOG protocol 33) and reported by Creasman. This study is required reading for physicians who care for patients with endometrial cancer. GOG protocol 33 was a surgical-pathologic study of 621 patients with clinical stage I endometrial cancer who were uniformly treated with total abdominal hysterectomy (TAH), bilateral salpingo-oophorectomy (BSO), peritoneal cytologic evaluation, and selective pelvic and paraaortic selected lymphadenectomy. Before this study, it was presumed most patients with endometrial cancer were at risk for nodal metastases, and nearly all patients required some form of pelvic radiation therapy (either preoperatively or postoperatively). The study demonstrated important relationships between pathologic factors and risk of nodal disease. For example, factors associated with nodal disease included higher tumor grade, deeper myometrial invasion, cervical involvement, (+) cytology, and LVSI. Because of this study, FIGO accepted a surgical-based staging system, including lymphadenectomy, of endometrial cancer in 1988.
| Stage | Patients ( n ) | 5-Year Survival (%) |
|---|---|---|
| Ia | 1063 | 91 |
| Ib | 2735 | 90 |
| Ic | 1219 | 81 |
| IIa | 364 | 79 |
| IIb | 426 | 71 |
| IIIa | 484 | 60 |
| IIIb | 73 | 30 |
| IIIc | 293 | 52 |
| IVa | 47 | 15 |
| IVb | 160 | 17 |
For endometrial cancer, FIGO staging reflects the progression of disease within the uterus and at extrauterine sites. Approximately 75% of patients with endometrial cancer present with disease limited to the uterus ( Table 5.7 ). For stage I disease, depth of tumor invasion into the myometrium is an important prognostic factor. The degree of myometrial invasion is a consistent indicator of tumor virulence ( Fig. 5.11 ) . DiSaia and associates noted that recurrences were directly related to depth of myometrial invasion in patients with stage I cancer treated primarily with surgery ( Table 5.8 ). The Annual Report of FIGO demonstrated a decrease in the survival rate as myometrial penetration increased ( Table 5.9 ). Lutz and coworkers determined that the depth of myometrial penetration was not as important as the proximity of the invading tumor to the uterine serosa. Patients whose tumors invaded to within 5 mm of the serosa had a 65% 5-year survival rate, but patients whose tumors were more than 10 mm from the serosa had a 97% survival rate. The depth of myometrial invasion is associated with the other prognostic factors, such as the grade of the tumor. As noted by DiSaia and associates, the survival rate of patients with poorly differentiated lesions and deep myometrial invasion is poor in contrast to that of patients who have well-differentiated lesions but no myometrial invasion. This suggests that virulence of the tumor may vary considerably, and as a result, therapy should depend on the combination of prognostic factors.
| Stage | Patients (%) |
|---|---|
| I | 3839 (73) |
| II | 574 (11) |
| III | 694 (13) |
| IV | 166 (3) |
| Endometrial only | 7/92 (8%) |
| Superficial myometrium | 10/80 (13%) |
| Medium myometrium | 2/17 (12%) |
| Deep myometrium | 15/33 (46%) |
| Stage | Patients | 5-Year Survival (%) |
|---|---|---|
| IaG1 | 698 | 93 |
| IbG1 | 1030 | 88 |
| IcG1 | 442 | 87 |
| IaG2 | 229 | 91 |
| IbG2 | 1307 | 93 |
| IcG2 | 485 | 84 |
| IaG3 | 66 | 75 |
| IbG3 | 280 | 82 |
| IcG3 | 247 | 66 |
Location of the tumor within the endometrial cavity is important because tumors low in the cavity may involve the cervix earlier than fundal lesions. The prognosis for women with cervical involvement (stage II) is worse than with stage I disease. Cervical involvement is often a surrogate marker for extrauterine disease spread or for risk of local recurrence. Data from GOG 33 showed that those with disease of the lower uterine segment had a higher incidence of pelvic lymph node metastases (16%) than do those with only fundal disease (8%). There is a similar frequency of paraaortic nodal metastases: a 16% incidence from disease of the lower uterine segment and a 4% incidence when only fundal disease is present. Previously, endocervical curettage (ECC) was commonly used to determine whether the patient had cervical involvement. Many false-positive results occur with this technique, however. In addition, the prognostic significance of endocervical glandular spread (formerly stage IIa) has been challenged. The new surgical staging adopted by FIGO in 2009 includes only patients with cervical stromal invasion in the stage II category. In some cases, cervical biopsy or ECC is required in the pretreatment planning of a patient with suspected cervical involvement.
Patients with stage III or IV disease may have a heterogeneous mix of disease characteristics. It is well recognized that endometrial cancer can and frequently does metastasize to the adnexa. Patients with adnexal or serosal involvement are categorized as having stage IIIA disease and are considered to have a higher risk of peritoneal recurrence. In GOG 33, 5% of patients had adnexal involvement. Adnexal metastasis was significantly associated with involved pelvic (32%) and paraaortic (20%) lymph nodes. In a large surgical trial comparing laparoscopy with open staging (GOG Lap 2 trial), 5% of 2616 patients enrolled were found to have stage IIIA disease (FIGO 1988 staging). In an analysis of 222 patients with clinical stage I carcinoma of the endometrium reported by DiSaia and colleagues, 16 (7%) were found to have metastasis in the adnexa. This finding correlated with many but not all of the other prognostic factors. Spread to the adnexa did not seem to be related to the size of the uterus. The grade of the disease did not appear prognostically important in regard to this in that 6% of patients with grade I tumors had adnexal disease compared with only 10% if poorly differentiated carcinoma was present. The depth of invasion did appear to be significant, however, in that only 4% of patients with only the endometrium involved had adnexal spread compared with 24% who had adnexal metastases if deep muscle was involved. If tumor was limited to the fundus of the uterus, only 5% of patients had disease in the adnexa; however, if the lower uterine segment or the endocervix was involved, one-third had spread to the adnexa. When metastasis was present in the adnexa, 60% of patients had malignant cells in the peritoneal cytologic fluid compared with only 11% if the adnexa were not involved. Recurrences appeared in only 14% of these individuals who did not have metastasis to the adnexa compared with recurrences in 38% of patients with adnexal metastasis.
Metastatic spread to lymph nodes (stage IIIC) has prognostic and therapeutic implications. It is clear that lymphadenectomy, to date, is the most sensitive way to identify nodal disease. The GOG 33 study demonstrated that as the depth of tumor invasion or tumor grade increased, the frequency of pelvic and paraaortic nodal metastases also increased ( Tables 5.10 and 5.11 ). For all 621 patients, 58 (9%) had positive pelvic nodes, and 34 (6%) had positive paraaortic nodes. Patients with grade 1, 2, and 3 tumors had pelvic nodal metastases of 3%, 9%, and 18% respectively. Similarly, patients with no myometrial invasion, inner one-third, middle one-third, and outer one-third invasion had 1%, 5%, 6%, and 25% pelvic nodal disease, respectively. The highest risk group included patients with grade III tumors and outer one-third invasion who had pelvic nodal involvement in 34% of cases ( Table 5.12 ). Without a lymphadenectomy, one may grossly estimate the probability of nodal involvement based on these data to select for or against the use of adjuvant therapy. This strategy potentially results in undertreatment or overtreatment of patients, however. In two large prospective randomized trials, routine lymphadenectomy resulted in the identification of more patients with nodal disease (9%–13%) compared with when only clinically suspicious nodes were removed (1%–3%). It is clear that patients with nodal disease have a poorer prognosis (3- to 5-year survival rate of 50%–75%) and different patterns of failure (nodal, distant) than patients with negative nodes (3- to 5-year survival rate of 80%–95%, vaginal cuff failures predominate). Patients with nodal involvement include those with pelvic nodal disease (IIIC1) or any paraaortic involvement (IIIC2). The substaging was created in 2009 given the belief of different outcomes associated with different levels of nodal involvement. The number of involved nodes and the extent of resection of grossly involved nodes also affect outcome. In addition, Mariani and McMeekin have suggested that patients with positive nodal disease plus other stage III defining features (positive cytology, adnexal or serosal involvement) have a much poorer prognosis than those with nodal disease only. Patients with stage IV or distant disease spread (intraperitoneal, lung, liver) have a particularly poor prognosis, with 5-year survival rate of less than 20%. The extent of surgical resection has been suggested to alter the prognosis in patients with advanced-stage disease. As with ovarian cancer, the extent of resection of disease reflects the biology of the tumor, aggressiveness of the surgery, and response to postoperative therapies.
| Grade ( n ) | Pelvic Nodes (%) | Aortic Nodes (%) |
|---|---|---|
| G1 (180) | 5 (3) | 3 (2) |
| G2 (288) | 25 (9) | 14 (5) |
| G3 (153) | 28 (18) | 17 (11) |
| Maximal Invasion ( n ) | Pelvic Nodes (%) | Aortic Nodes (%) |
|---|---|---|
| Endometrium only (87) | 1 (1) | 1 (1) |
| Superficial muscle (279) | 15 (5) | 8 (3) |
| Intermediate muscle (116) | 7 (6) | 1 (1) |
| Deep muscle (139) | 35 (25) | 24 (17) |
| Depth of Invasion | Grade | ||
|---|---|---|---|
| Grade I ( n = 180) | Grade II ( n = 288) | Grade III ( n = 153) | |
| Endometrium only ( n = 86) | 0%/0% | 3%/3% | 0%/0% |
| Inner third ( n = 281) | 3%/1% | 5%/4% | 9%/4% |
| Middle third ( n = 115) | 0%/5% | 9%/0% | 4%/0% |
| Outer third ( n = 139) | 11%/6% | 19%/14% | 34%/23% |
Tumor Grade
The degree of histologic differentiation (tumor grade) of endometrial cancer is a sensitive indicator of prognosis and is included in FIGO stage assignment. The Annual Report on the Results of Treatment in Gynecological Cancer has evaluated survival in regard to grade in patients with clinical stage I adenocarcinoma of the endometrium ( Table 5.13 ). Tumor grade is inversely related to survival decreases; as grade increases, survival is poorer. In their review of 244 patients with stage I disease, Genest and colleagues noted that patients with grade I disease had a 5-year survival rate of 96%. This dropped to 79% and 70% for grade II and grade III, respectively. Data presented by Morrow for outcomes in patients enrolled on GOG 33 show that recurrence-free survival markedly diminishes with increasing grade, with progression-free survival (PFS) at 48 months being approximately 95%, 85%, and 68% for grade I, II, and III tumors, respectively. Grade of tumor also correlates with other factors of prognosis. Table 5.14 shows the relationship between differentiation of the tumor and depth of myometrial invasion as reported by Creasman from the GOG 33 study. As the tumor becomes less differentiated, the chances of deep myometrial involvement increase. However, exceptions can occur: Patients with a well-differentiated lesion can have deep myometrial invasion, but patients with a poorly differentiated malignant neoplasm might have only endometrial or superficial myometrial involvement.
| Grade | Survival ( n = 5017) (%) |
|---|---|
| 1 | 91 |
| 2 | 90 |
| 3 | 81 |
| Myometrial Invasion | Grade | ||
|---|---|---|---|
| 1 (%) | 2 (%) | 3 (%) | |
| None | 24 | 11 | 11 |
| Superficial | 53 | 45 | 35 |
| Mid | 12 | 24 | 16 |
| Deep | 10 | 20 | 42 |
Lymphovascular Space Involvement
Hanson and colleagues described 111 patients with stage I endometrial cancer and found capillary-like space (CLS) involvement in 16. This was most frequently found in patients with poorly differentiated tumors with deep invasion. These patients had a 44% recurrence rate compared with 2% if the CLS was not involved. This was an independently significant prognostic factor. In the GOG study of 621 patients, it was shown that 93 (15%) had CLS involvement. The incidences of pelvic and paraaortic node metastases were 27% and 19%, respectively. This compares with a 7% occurrence of pelvic node metastasis and a 3% occurrence of paraaortic node metastasis when there is no CLS involvement. In risk models predicting risk of recurrence in node-negative, early-stage endometrial cancer, the GOG has suggested that LVSI is an important risk factor. In the Post-Operative Radiation Therapy in Endometrial Cancer (PORTEC) trial, LVSI was considered a factor associated with distant site of failure.
Tumor Size
Schink and coworkers evaluated tumor size in 91 patients with stage I disease. The incidence of lymph node metastases in patients with tumor size less than 2 cm was only 6%. If the tumor was larger than 2 cm in diameter, there were nodal metastases of 21% and up to 40% if the entire endometrium was involved. Patients with lesions greater than 2 cm in size and less than half myometrial invasion had no nodal metastasis. Using multivariate analysis, the authors showed that tumor size was an independently significant prognostic factor. Watanabe and associates did not find cancer size was predictive of lymph node metastasis. Gynecologic oncologists from the Mayo Clinic have developed a risk model predicting nodal metastases and have suggested that grade I and II tumors that are smaller than 2 cm are particularly low risk for nodal disease.
Peritoneal Cytology
The importance of peritoneal cytology in endometrial cancer is controversial. In GOG 33, 76 (12%) had malignant cells identified by cytologic examination of peritoneal washings. Of these patients, 25% had positive pelvic nodes compared with 7% of patients in whom no malignant cells were found in peritoneal cytologic specimens ( P >0.0001). It is true that peritoneal cytology, to a certain degree, mimics other known prognostic factors—that is, if peritoneal cytologic specimens are positive, other known poor prognostic factors may also be identified. In addition, data from GOG 33 also suggested that the RR for recurrence with positive cytology was 2.4 ( P = 0.02).
Given the relationship of positive cytology with other known risk factors, it is important to evaluate data sets coming from patients who undergo complete surgical staging. Saga and colleagues reported on a series of 307 (32 with positive cytology, 275 with negative cytology) patients with endometrioid-type cancer, all who underwent complete staging and had negative lymph nodes. The authors reported that 5-year survival rate was 87% with positive cytology compared with 97% with negative cytology. Most patients with positive cytology received chemotherapy postoperatively. Havrilesky et al. reported on experience from Duke University. Patients with positive cytology alone ( n = 37) were compared with patients with adnexal or serosal involvement ( n = 20). The 5-year survival rate was similar between the groups (62% positive cytology, 68% adnexal or serosal disease), but in a multivariable analysis, cytology was an independent predictor of recurrence and poorer survival. This study included patients with nonendometrioid histologies, and lymphadenectomy was not routinely performed. In another review of the literature, Wethington demonstrated that in endometrioid-type tumors with low risk factors (superficial invasion, grade I or II); the recurrence rate was only 4%. By comparison, 32% of patients with higher grade tumors or deeper invasion and positive cytology had a recurrence. These results suggest that for at least a subset of cytology patients, the risk of recurrence approximates that driven by other uterine factors (depth of invasion, tumor grade). In 2009, FIGO dropped cytology as a stage IIIA defining characteristic.
Milosevic and colleagues reviewed 17 studies. In 3820 patients, the prevalence of positive cytologic findings was 11%. The three largest studies totaling more than 1700 patients (Haroung and associates, Turner and colleagues, Morrow and coworkers) using multivariate analysis noted that the finding of malignant cells on cytologic examination was independently significantly associated with either recurrence or reduced survival. Pooled odds ratio for the entire series was 4.7 (CI, 3.5–6.3) for disease recurrence. All studies note the highest correlation of malignant cytologic specimens with extrauterine disease. It does appear that with multivariate analysis, the presence of malignant cells is an important prognostic factor even when disease is limited to the uterus.
Optimal therapy has not been determined to date. Historically, intraperitoneal 32 P or oral progestins have been used to manage disease in patients with positive cytology. Today, uterine risk factors are largely used to define postoperative adjuvant therapy independent of cytology status and, increasingly, in otherwise low-risk patients (endometrioid-type tumors, negative nodes), observation is often considered. Chemotherapy has also been used in the adjuvant setting for patients with positive cytology.
Molecular Indices
Hormone receptors.
Historically, estrogen and progesterone receptors were the first “targets” describing the molecular biology of endometrial cancer. Using multivariate analysis to analyze hormone receptor status, Creasman and associates noted that in stage I and stage II cancers, progesterone receptor–positive status was a highly significant, independently prognostic factor in endometrial cancer. Without progesterone receptor status in the model and with the evaluation of estrogen receptor status in its stead, estrogen receptor–positive status was an independent prognostic factor but not to the degree of progesterone receptor–positive status. Hormone receptor status may closely mirror grade, however.
Today there is an explosion of research into the molecular makeup of endometrial cancer. Cytogenetic studies have described gross chromosomal alterations, including changes in the number of copies of specific chromosome. The extent of abnormalities in a given tumor is relatively low. About 80% have normal diploid DNA content. Aneuploidy in 20% is usually associated with high-grade, extrauterine disease, high-risk histotypes, and poor prognosis. So-called loss of heterozygosity occurs at a relatively low frequency compared with other solid tumors. When chromosomal loss of heterozygosity does occur, underlying molecular genetic defects have been observed on 17p and 10q that correlate with mutational inactivation of TP53 and PTEN, respectively. Individual tumors with a greater number of gains and losses are associated with a poorer prognosis, and some changes seen in cancer are also present in atypical hyperplasia but not simple hyperplasia lesions.
Mutational activation or aberrant expression of some oncogenes has been described but to a lesser degree than tumor suppressor genes. The RAS gene family is the most commonly identified oncogene aberration in human cancers and is present in 10% to 30% of endometrial cancers. This mutation appears to occur early in the neoplastic process, and the incidence is the same in endometrial hyperplasia. Correlation of RAS mutation to survival has produced conflicting results. About 10% to 15% of endometrial cancer has overexpression of ERBB-2 (HER2/neu) protein. Overexpression appears to be confined to high-grade or advanced-stage tumors.
The FMS oncogene encodes a tyrosine kinase, which serves as a receptor for macrophage colony-stimulating factor (m-CSF). Expression of FMS correlates with advanced-stage, high-grade, and deep myometrial invasion. Expression of C-MTC, which has been observed in normal endometrium and endometriosis, has a higher expression in secretory endometrium. Several studies suggest amplification is present in a fraction of endometrial cancers.
Mutation of TP53 tumor suppressor gene, the most common genetic abnormality currently recognized in human cancers, is present in 10% to 30% of endometrial cancers. Overexpression or mutation (or both) is associated with prognostic factors. In a study of more than 100 endometrial hyperplasia specimens, TP53 mutation was not present. PTEN mutation analysis in endometrial cancer indicates that this gene is somatically inactivated in 30% to 50% of all tumors, the most frequent molecular genetic alteration defined in endometrial cancer. There does appear to be a correlation between microsatellite instability and PTEN mutation. PTEN mutation is observed in 20% of endometrial hyperplasias, suggesting that this is an early event in the development of some type I endometrial cancers.
Inherited mutations in gene encoding DNA mismatched repair proteins, primarily MSH2 and MLM1, are responsible for HNPCC, for which endometrial cancer is the second most common cancer in women with these mutations. Cancers in these individuals are characterized by frame shift mutations in multiple microsatellite repeat sequences throughout the genome. This instability is also seen in 20% of sporadic endometrial cancers. In these sporadic cancers, acquired mutation in mismatched repair genes is rare. Endometrial cancers that exhibit microsatellite instability tend to be type I, which has a more favorable prognosis. This microsatellite instability is present in some cases of complex hyperplasia associated with endometrial cancer but is not seen in UPSC cancers.
Type I endometrial cancers are commonly described to include tumors seen in obese and nulliparous women, are well differentiated, are superficially invasive, and frequently carry a good prognosis. These tumors also share several common molecular changes and tend to have the following genetic features: diploid, low allelic imbalance, K-RAS, MLH1 methylation, and PTEN. In contrast, type II with poor prognostic pathologic features have aneuploid, high allelic imbalance, K-RAS, TP53, and HER2/neu changes.
Recently, array-based technology has allowed a more comprehensive characterization of endometrial cancers. It should be noted that these new technologies are in their infancy, although multiple papers using these techniques have been reported, many with DNA microassay. The effects of exogenous PTEN expression in endometrial carcinoma cell lines lacking PTEN function has been studied by Matsushima-Nishiu and associates. They observed increased expression in 99 genes and repression of 72 genes, many of which are known to be involved in cell proliferation, differentiation, and apoptosis, suggesting the potential power of expression profiling identifying molecular pathways affected by critical cancer-related genes. Proteomic profiling, which is the study of intact and fragmented proteins and their function, is being evaluated. Newer technologies allow the creating of proteomic fingerprints that reflect in serum what is happening in the end organs. The biochip is playing a major role in this evaluation. As little as 1 µL of serum can be evaluated, and this technology is very sensitive to low molecular weight protein regions. The results of The Cancer Genome Atlas (TCGA) Research Network has further improved our understanding of the molecular characteristics of endometrial cancer. They described four molecular subtypes, including (1) POLE (ultra-mutated) tumors, (2) microsatellite unstable tumors, (3) copy-number high tumors with mostly TP53 mutations, and (4) a remaining group without these alterations. For protocol 210, the GOG has collected material (tissue, serum, urine) on a large number of endometrial cancer patients stored in its tumor bank for in-depth research using these newer technologies, which will hopefully allow us to understand the malignant process.
Correlation of Multiple Prognostic Factors
The GOG 33 protocol identified the extent of disease spread identified at surgery correlated with outcomes. Although single factors were associated with recurrence, the combination of factors could also establish risk. For example, Morrow showed that patients with no risk factors (LVSI, cervical involvement, adnexal involvement, nodal disease) had a very low risk of recurrence, but 20% with one, 43% with two, and 63% with three or more factors recurred. In multivariate analysis, patients with disease limited to the uterus were at increased risk for recurrence if there were deep myometrial invasion, vascular space involvement, or positive washings. Fig. 5.11 is the author’s attempt to compartmentalize these risk factors into risk categories for predicting prognosis and guiding decisions for adjuvant therapy. The lines between categories are somewhat porous, consistent with most clinical situations.
Data from three randomized trials evaluating the use of postoperative pelvic radiation therapy have been useful in shaping models predicting risk of recurrence after surgery. Aalders identified a subset of the 95 of 540 (18%) patients enrolled in a randomized trial of vaginal cuff brachytherapy with or without pelvic radiation therapy with grade III tumors and greater than 0% myometrial invasion to be particularly notable for risk of recurrence. Patients with these factors had a pelvic recurrence rate of 20% with vaginal brachytherapy alone compared with 5% with the combination of pelvic and vaginal radiation. It is interesting that approximately 15% of patients with these tumor characteristics failed at a distant site, regardless of radiation technique. The PORTEC trial compared pelvic radiation therapy with observation after hysterectomy without lymph node dissection in patients with endometrial cancer. In this trial of 714 patients, a subset of patients with grade III tumors (10%), age older than 60 years (72%), or greater than 50% depth of invasion (59%) was identified in which having two of three factors defined a high-risk group. The locoregional failure rate was 23% in this group who underwent surgery followed by observation compared with 5% after pelvic radiation therapy. There was no difference in distant sites of recurrence (~5% each group) or in cancer-related deaths (8% observation, 11% radiation). The GOG conducted a randomized trial (GOG 99) of observation versus pelvic radiation in a group of patients with surgically documented negative nodes and any amount of myometrial invasion. Depending on patient age and number of risk factors (LVSI, grade I or II tumor, outer one-third myometrial invasion) ( Table 5.15 ), a high-intermediate (H-IR) risk group could be identified. The H-IR group accounted for one-third of all patients enrolled but two-thirds of recurrences. Perhaps equally important was the identification that two-thirds of the patients enrolled who did not have H-IR features had an incredibly low risk of recurrence (2%–3%), suggesting that in addition to defining high-risk groups, low-risk groups that may avoid postoperative adjuvant therapy may also be identified.
| Age | Risk Factor |
|---|---|
| Any age + 3 factors | LVSI |
| ≥ 50 years + 2 factors | Grade II or III tumor |
| ≥ 70 years + 1 factor | Outer one-third invasion |
The value of risk models is that they can be applied to an individual patient incorporating all of the information (age, uterine characteristics) to provide a reasonable estimate of risk of recurrence and probability of benefit of selected adjuvant therapy. Research is under way to identify molecular markers that may augment clinical-pathologic information in establishing risk. Other cancer types (breast, prostate, bladder) have well-validated disease-specific nomograms predicting risk and benefit to adjuvant therapy. Similarly, developed and validated models for endometrial cancer are needed.
Treatment
Surgical Management of Endometrial Cancer
We prefer to sample the endometrium in symptomatic postmenopausal patients as the first diagnostic technique. If histologic findings are “negative,” the patient is observed. A D&C is done only if the patient continues to be symptomatic after the negative biopsy result. After a tissue diagnosis of endometrial cancer is established, the patient should be assessed for surgical options of therapy. Most patients require routine blood counts (CBC), metabolic profile, and a metastatic evaluation with chest radiography. Routine use of computed tomography (CT) or MRI scans has not shown to be useful and should be reserved for unusual situations (high-risk tumor types, evidence of intraperitoneal disease, positive chest radiograph). Most patients with endometrial cancer are candidates for definitive surgery, including surgical staging (lymphadenectomy). Routes of hysterectomy include vaginal, abdominal, laparoscopic (total or assisted), and robotic and have broadened the surgical options for patients.
The surgical evaluation of most patients with endometrial cancer requires a thorough inspection of the peritoneal cavity, collection of cytologic washings, and pelvic and paraaortic lymphadenectomy (PALA) in addition to hysterectomy. Cytologic evaluation of peritoneal fluids, or washings, has been a common surgical step in staging endometrial cancer because of associations with extrauterine disease and prognosis. After the peritoneal cavity is opened, an assessment of the amount of peritoneal fluid in the pelvis is made. If none is present, 100 to 125 mL of normal saline solution is injected into the pelvis. This can be done easily with a bulb syringe. The saline solution is admixed in the pelvis, withdrawn with the syringe, and sent for cytologic evaluation. We continue to recommend that peritoneal cytologic evaluation be performed in all patients undergoing surgery for endometrial cancer, although FIGO no longer uses cytology as a stage-defining characteristic. Omental biopsy may be considered in patients with gross spread to the omentum or adnexa or in cases with high-risk histologies such as serous or clear cell tumors. Approximately 10% of all patients will be found with nodal metastasis when surgically staged, but only 1% to 3% will be found to have nodal disease if nodes are evaluated only when clinically suspicious. In a large GOG study of 621 staged patients, 6% of patients with clinical stage I disease were noted to have intraperitoneal disease.
The definitive treatment for patients with endometrial cancer is hysterectomy. The hysterectomy should be extrafascial, and removal of the upper vagina does not appear to decrease vault recurrences ( Fig. 5.12 ). Extirpation of the uterus removes the primary tumor and can provide important information that can be used to estimate risk of spread to lymph nodes or risk of recurrence. Removal of the adnexa is thought to be important given that approximately 5% of endometrial cancers have metastatic disease to the ovaries, fallopian tubes, or both. In addition, synchronous ovarian and endometrial cancers are not infrequent, particularly in younger patients. Historically, total abdominal hysterectomy (TAH) and bilateral salpingo-oophorectomy (BSO) have been the hallmarks of therapy for endometrial cancer. There are no data to suggest that the route of hysterectomy influences recurrence-free or overall survival (OS) outcomes for patients; as such, the hysterectomy (abdominal, laparoscopic, and robotic) is the safest and facilitates rapid recovery and thus is suggested.
Several studies note the role of vaginal hysterectomy in highly selected patients with endometrial cancer for whom surgical staging cannot be performed safely (morbid obesity, significant cardiopulmonary medical comorbidities, advanced age). Because factors associated with the choice of vaginal hysterectomy (morbid obesity, medical comorbidities) are often associated with favorable uterine characteristics (low-grade lesions, smaller uterus), it is not surprising that survival rates are comparable to those of the abdominal approach. Chan and coworkers reviewed 51 medically compromised patients treated with vaginal hysterectomy and reported 3- and 5-year disease-specific survival rates of 91% and 88%, respectively. Of note, approximately 50% of women could not have the ovaries removed at the time of vaginal hysterectomy. Smith evaluated 63 patients with obesity or medical comorbidities and found that vaginal hysterectomy was safe and well tolerated in this patient population. Vaginal hysterectomy may represent a reasonable tradeoff for patients who may not tolerate other approaches or for whom surgical staging is not being considered (atypical hyperplasia, some patients with grade I cancers).
Over time, there has been a more widespread use of lymphadenectomy in the management of patients with endometrial cancer. Lymph node dissection provides the best estimate of spread of disease (vs. palpation or use of imaging studies), the lymph node status is prognostically important (as evident from the different survival rates seen with different stages of disease), and patients who are found to have positive or negative nodes receive different postoperative therapy vs. patients with unknown status of lymph nodes. In a joint statement by ACOG and the Society of Gynecologic Oncologists (SGO) in 2005, both organizations suggested that most women with endometrial cancer should undergo systematic surgical staging, including bilateral pelvic and PALA. Today, debate continues as to which patients (all, none, or some) benefit most from lymphadenectomy and the technique (pelvic, pelvic and paraaortic, level of paraaortic dissection) that should be performed.
The extent of the lymph node dissection is thought to be important. Early studies were conducted with selective lymphadenopathy or lymph node sampling (only visibly enlarged lymph nodes are selectively removed). Some data suggest that a true lymphadenectomy (complete skeletonization of vessels) should be performed. It seems intuitive that a sufficient number or distribution of nodes needs to be removed to represent an adequate sampling. When lymphadenectomy is done, the retroperitoneal spaces in the pelvis are opened in routine fashion. The vessels are outlined, and the lymph node–bearing tissue along the external iliacs from the bifurcation to the inguinal ligament is removed. The obturator fossa anterior to the obturator nerve is cleaned of lymphoid tissue. Lymph nodes along the common iliacs are also removed. The left and right paraaortic nodes are approached by retracting the small intestine into the upper abdomen and incising the peritoneum over the upper common iliac artery and lower aorta. The main vessels are outlined, and the ureter is retracted laterally. On the right, the tissue overlying the vena cava and the aorta are removed en bloc, beginning at the bifurcation of the aorta and extending caudad. On the left, the left common nodes are frequently quite lateral. Using this technique, one should have a total of 20 to 30 pelvic and paraaortic lymph nodes available for histologic evaluation. The upper limit of the dissection (unless enlarged nodes are noted above this area) is usually the inferior mesenteric artery (IMA), but some surgeons suggest that the dissection should extend to the level of the renal vessels.
The extent of nodal dissection to include paraaortic nodes is also thought to be important in endometrial cancer. There appear to be two nodal drainage basins—pelvic and paraaortic. Studies indicate that when lymph nodes are positive, approximately 50% to 60% of the time paraaortic nodes are involved. In a retrospective study from the Mayo Clinic, 137 patients at high risk for nodal involvement who underwent PALA (PAL+) were compared with those who did not undergo surgical evaluation of the paraaortic nodes (PAL−). The 5-year survival rate was 85% for PAL+ patients compared with 77% for PAL− patients. In 51 patients with pelvic or paraaortic node metastasis, survival was 77% for PAL+ patients compared with 42% in the PAL− group. It is generally thought that the rate of isolated paraaortic lymph node metastasis is less than 5%; metastasis isolated to the paraaortic lymph node and metastasis above the IMA are seen. Mariani and colleagues reported on a study of 482 patients with endometrial cancer at the Mayo Clinic. A total of 281 underwent lymphadenectomy to the renal vessels, and 22% had positive lymph nodes; of these, 51% had both positive pelvic and paraaortic nodes, 33% had positive pelvic nodes only, and 16% had isolated involvement of the paraaortic nodes. Of note, 46% of those with isolated paraaortic lymph nodes were only positive above the IMA, and 77% had at least one metastatic node located above the level of the IMA. However, the authors do report that there were no positive lymph nodes detected in those with grade I or II tumors with a tumor size of smaller than 2 cm with less than 50% invasion, raising the question of the benefit of lymphadenectomy in the truly low-risk group. Abu-Rustum and colleagues demonstrated a 1% risk of isolated paraaortic lymph node metastasis in both the low- and high-grade tumors. Based on these and other reports, PALA does have an important role in comprehensive staging. For high-risk patients, the data suggest even a therapeutic role for paraaortic lymph node dissection. However, prospective trials are needed to confirm this concept for patients with early-stage endometrial cancer with high-risk features.
Since 1988 when FIGO changed endometrial staging from clinical to surgical, there have been questions raised as to whether the lymphadenectomy is only diagnostic, which is an important determinate, or whether it also could be therapeutic. Kilgore and associates, in evaluating 649 patients, noted that those who underwent multiple-site lymph node removal had significantly better survival rates than those patients who had no lymph nodes removed ( Figs. 5.13 and 5.14 ). Lymph node removal resulted in a better survival rate than for those without lymph node removal plus postoperative radiation. Cragun and colleagues reported that patients with grade III or poorly differentiated cancers with more than 11 lymph nodes removed had an improved survival (hazard ratio [HR], 0.25) and PFS (HR, 0.26) compared with those with fewer than 11 lymph nodes removed. However, the number of lymph nodes removed was not predictive of survival outcome in those with grade I and II tumors. This association between lymphadenectomy and improved survival remained when controlling for adjuvant radiation treatment, emphasizing the impact of extensive lymph node dissection. A retrospective review by Chan and colleagues of more than 12,000 patients showed an increased 5-year survival rate for the intermediate- and high-risk group that underwent an extensive lymph node dissection. However, there was no benefit of nodal dissection seen in the low-risk group.
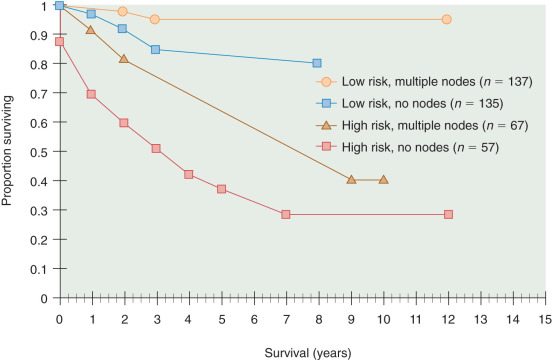
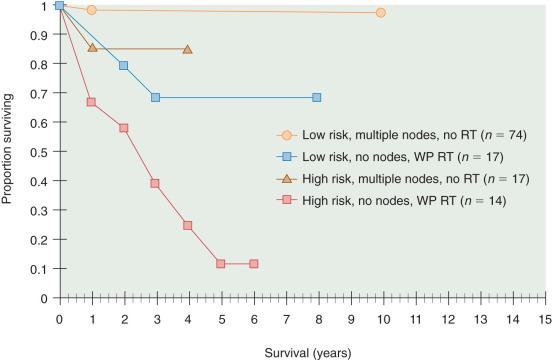
Removal of nodes involved by tumor has been supported as a therapeutic option. In a study reported by Havrilesky et al., 91 patients were identified with stage IIIc disease. There were 39 with microscopic involvement of the lymph nodes and 52 with grossly enlarged nodes. After surgery, 92% received some type of adjuvant therapy with 85% receiving radiation therapy. The survival rate (5 years) was 58% in the 39 with microscopic lymph nodes, 48% in 41 patients with grossly positive lymph nodes completely resected, and only 22% in the 11 with unresected lymph nodes. The authors believed that these data suggested a therapeutic benefit for lymphadenectomy. Similarly, in a small study of 41 patients, Bristow and coworkers noted disease-free survival was improved if patients with bulky adenopathy underwent complete resection of involved nodes compared with patients who had gross residual disease in lymph nodes remaining after surgery (37.5 vs. 8.8 months; P = 0.006). Onda and colleagues carried out thorough pelvic and paraaortic lymphadenectomies on 173 patients with stage I to III endometrial cancer. The average numbers of lymph nodes removed were 38 pelvic and 29 paraaortic. There were 30 patients (17%) with positive nodes: 10 to pelvis only, two paraaortic only, and 18 with metastasis to both pelvic and paraaortic nodes. Selected patients received radiation therapy with extended fields, combination chemotherapy, or both. In the 10 patients with only pelvic metastasis, the 5-year survival rate was 100%; it was 75% in those with paraaortic involvement. The authors suggest that, although postoperative treatment may contribute to these excellent results, systematic pelvic and PALA was a contributing factor.
One of the important effects of lymph node dissection is that it identifies that most patients with negative lymph nodes are at very low risk for recurrence. As such, lymph node dissection has allowed for modifications of postoperative therapy away from pelvic radiation therapy. In one study, Mohan and associates evaluated 159 stage I patients who had full pelvic lymphadenectomy (PLA) and received vaginal brachytherapy rather than the more traditional pelvic radiation. In general, vaginal brachytherapy is a quicker therapy (1–3 days vs. 5–6 weeks for pelvic radiation) and is associated with fewer side effects. In the Mohan study, the 15-year OS was 92%, and the recurrence rate was 4%, all at distant sites. Podratz and associates reviewed four studies that used thorough lymphadenectomies in moderate- and high-risk patients who did not receive postoperative radiation therapy. There were 20 recurrences (7%) in 305 patients; only five recurrences were local or regional, with four being in the vagina. Those four did not receive postoperative brachytherapy but were salvaged with subsequent radiation.
The disadvantages of a lymph node dissection include that the performance of lymph node dissection requires a specially trained surgeon, has the potential to increase complications (postoperative ileus, lymphedema), and has not been prospectively validated to improve outcomes. Two recent phase III trials compared the use of lymph node dissection or not in patients with endometrial cancer. In a large multicentric study (ASTEC study), 1408 patients were randomly assigned to abdominal hysterectomy or bilateral salpingo-oophorectomy with or without PLA. A median follow-up period of 37 months was obtained. There was no difference in survival between the two groups. This was an intent-to-treat study and has been criticized as having a relatively large noncompliance in regard to lymphadenectomy and subsequent therapy. In the lymphadenectomy arm, almost a half had no nodes or a small number (≤9) removed. This study was further complicated by the fact that many of these patients were secondarily randomly assigned into a postoperative radiation study, not taking into consideration surgical findings. PALA was not the standard management, although some patients had these nodes removed.
The second study was published by Pierluigi Benedetti Panici. The Italian study randomly assigned 514 stage I patients to systematic PLA or no lymphadenectomy. Although 13% of patients in the lymphadenectomy arm had known metastasis versus 3% in the no-lymphadenectomy arm, there was no difference in recurrence or OS. The protocol required a minimum of 20 lymph nodes to be removed; however, the lymphadenectomy was limited to the pelvis, although PALA could be removed at the discretion of the surgeon. Postoperative radiation was left to the discretion of the treating physician. Adjuvant therapy was similar in the two groups. Whether or not this had an effect on survival is unknown because this was not standardized.
A study from Japan (SEPAL study) was published in early 2010. This was a study of PLA ( n = 325 patients) versus PALA ( n = 346 patients) in endometrial cancer. It was not a randomized study; however, one hospital did only pelvic nodes and the other pelvic and paraaortic. Lymphadenectomy was certainly adequate for using the number of lymph nodes removed as a parameter. The survival rate was significantly better ( P >0.001) in women receiving the PLA and PALA. This was seen in intermediate- and high-risk patients but not in low-risk patients. In a multivariant analysis, the PLA plus PALA reduced the risk of death compared with PLA only. In the high-risk group, patients receiving chemotherapy plus PLA plus PALA had a better survival than patients receiving chemotherapy plus PLA only. PLA plus PALA and adjuvant chemotherapy were independently associated with improved survival.
The Cochrane Database subsequently concluded that there was no overall statistical difference between PFS and OS in patients who had a lymphadenectomy versus those who did not but did report more morbidity for those patients who had a lymphadenectomy. Cochrane only considered the ASTEC and the Italian study in reaching this conclusion. The extent of the lymphadenectomy appears to be critical in evaluating studies in determining an appropriate surgical procedure for patients with endometrial cancer. Multiple studies confirm that both pelvic and paraaortic lymph nodes are at risk for metastasis.
Patients with stage II carcinoma of the endometrium, because of extension of disease into the endocervix, will have a greater propensity for lymph node metastasis. For example, in the GOG 33 protocol, 16% of patients with cervical involvement had positive pelvic lymph nodes versus 8% with a fundal location of tumor. Even in patients with occult cervical involvement, 19% of patients had lymph node metastases. In addition, parametrial and vaginal involvement are thought to be more common when the cervix is involved. As such, surgery in the form of radical hysterectomy and lymphadenectomy has been advocated when there is gross cervical involvement with tumor. Because many cases of cervical involvement are occult and only recognized after surgery, simple hysterectomy with lymphadenectomy appears to be adequate surgery in most cases. Postoperative radiation therapy can be planned, depending on surgical-pathologic findings, including use of pelvic radiation or vaginal cuff brachytherapy, both, or neither.
In 1993, Childers and others pioneered laparoscopic lymph node removal in conjunction with vaginal hysterectomy and bilateral salpingo-oophorectomy. In the hands of those who acquired those surgical skills, the outcomes appeared comparable, and some advantages were gained, such as short hospitalization and rapid postoperative recovery. A large GOG study comparing the laparoscopic approach versus laparotomy has been completed. Walker and colleagues published the results of GOGLAP2, which randomly assigned patients between laparoscopic versus open laparotomy surgical staging. Laparoscopy was initiated and completed without conversion in 74%. Conversion from laparoscopy to laparotomy was secondary to poor visibility in 15%, metastatic cancer in 4%, and bleeding in 3%. Patients randomly assigned to undergo laparoscopy had significantly fewer moderate to severe postoperative (14% vs. 21%) complications and similar rates of intraoperative complications. Length of hospital stay was significantly shorter for those randomized to undergo laparoscopy (median 3 days vs. 4 days), but operative time was significantly longer (median, 204 minutes vs. 130 minutes). Pelvic and paraaortic nodes were removed in 92% of patients undergoing laparoscopy and 96% of patients undergoing laparotomy, and cytology was performed in 96% vs. 98%. Neither treatment arm demonstrated an improved ability to detect metastatic disease. Quality-of-life evaluation found a better body image and return to normal activities for the patients undergoing laparoscopy. Laparoscopic surgical staging is feasible and safe for patients with uterine cancer and results in fewer complications and shorter hospital stays. A reduction in paraaortic node evaluation is a potential risk of laparoscopic staging. Recurrence rates were lower than anticipated; the estimated 3-year recurrence rate was 11% with laparoscopy and 10% with laparotomy, and the estimated 5-year OS rate was almost identical in both arms (90%). Today, advancements of laparoscopic surgery, including use of a robotic surgical platform, have been suggested as the next step. Robotic surgery offers three-dimensional graphics, improved ergonomics, and greater dexterity of surgical instruments. Robotic surgery may extend the applications of laparoscopy, especially in obese patients or patients in whom a vaginal component is more difficult. However, no randomized trials have confirmed equivalence or superiority of robotic assistance.
Surgical staging has now been accepted as standard therapy in patients with endometrial cancer unless clinical conditions suggest otherwise. Of the 6260 patients with endometrial cancer reported to the last Annual Report of FIGO, 94% were surgically staged. It is appreciated that institutions reporting to the Annual Report are academic ones and that most patients with endometrial cancers, at least in the United States, have their primary surgery at academic hospitals. Because endometrial cancer staging has been determined by surgical staging, it has been suggested by some that these are patients who are at low risk (ie, grade I) for lymph node metastasis and that lymphadenectomy is not worthwhile. Increasing data suggest that even in grade I disease, as noted on endometrial biopsy, a significant number of patients have full surgical staging findings that would have an impact on further therapy. Ben-Shachar and colleagues, in evaluating 181 grade I endometrial cancers, found that 19% had grade change on hysterectomy specimen, 11% had extrauterine disease, 4% had lymph node metastasis, and 26% on final evaluation had high-risk intrauterine factors. Of note, the authors believed because of full surgical staging that 12% needed and received adjuvant therapy, and 17% who may have received postoperative treatment did not based on full surgical findings. Geisler and associates found in 349 patients that of those with grade I lesions, 16% had positive nodes and 3% had positive paraaortic nodes only. Of all positive nodes, 31% occurred in grade I lesions. As a result of these and other studies, many believe that all patients with endometrial cancer should have the benefit of full surgical staging, which includes peritoneal cytology, bilateral pelvic lymphadenectomy and PALA, and total abdominal hysterectomy and bilateral salpingo-oophorectomy. Obviously, complete evaluation of the entire peritoneal cavity and its contents should be performed, and any suspicious areas should be pathologically evaluated.
Straight and associates reported on a large number of patients who were surgically staged. Low-risk factors, stage IaG1 and 2, were present in 103 patients. None received postoperative therapy, and none had recurred. Intermediate risk was defined as stage IaG3 and all stage I and Ic cancers. There were 440 patients of whom 93% received no further therapy. Twenty-eight patients received postoperative therapy, and one (4%) had a recurrence compared with 5% of those not radiated. The latter patients received therapy at the time of recurrence, and 62% were successful. The Annual Report (2003) noted 5-year survival rates with surgery only of 93%, 91%, 73%, 79%, and 73%, respectively, for stages Ia, Ib, Ic, and IIa, and IIb. This compares with 89%, 91%, 83%, 83%, and 75%, respectively, if surgery plus postoperative radiation is used. There is a suggestion that in stage Ic, the survival rate is somewhat better when postoperative radiation is added than surgery alone. What factors went into decision making regarding postoperative therapy is unknown. In a multi-institutional study, 220 surgically staged Ic patients were identified. High-risk histotypes were excluded. Adjuvant radiation was used in 99 (45%), 56 brachytherapy only, and 19 whole-pelvis radiation, and 24 received both. OS rates between those treated with surgery and surgery plus radiation was similar (92% vs. 90%).
Radiation Therapy
Radiation therapy has been the mainstay of adjuvant therapy for endometrial cancer. Historically, nearly all patients received some form of preoperative or postoperative radiation therapy given the perception of risk for recurrence was high. Whereas GOG 33 defined relationships between depth of invasion and tumor grade and extrauterine spread after surgical staging, it also showed that most patients were not at risk for extrauterine disease spread. As such, the routine use of pelvic radiation therapy became increasing questioned. To date, four large prospective randomized studies have compared external pelvic radiation with observation or vaginal brachytherapy in women with endometrial cancer ( Table 5.16 ). Aalders and colleagues compared vaginal cuff brachytherapy with or without pelvic radiation in 540 patients with early-stage endometrial cancer. Patients did not undergo lymph node dissection. The addition of pelvic radiation therapy reduced local failures (2% vs. 7%), but there was no difference in 5-year survival rate between the groups (89% pelvic, 91% brachytherapy). Onsrud et al. updated the long-term follow-up for these patients. After a median 20.5 years of follow-up, no statistically significant difference was revealed in OS rate between treatment groups. However, women younger than age 60 years had significantly higher mortality rates after external-beam radiotherapy (EBRT) (HR, 1.36; 95% CI, 1.06–1.76) than the control group. The risk of secondary cancer increased after EBRT, especially in women younger than age 60 years (HR, 2.02; 95% CI, 1.30–3.15). The authors concluded that there was no survival benefit of external pelvic radiation in early-stage endometrial carcinoma. In women younger than age 60 years, pelvic radiation decreased survival and increased the risk of secondary cancer. Thus, adjuvant EBRT should be used with caution, especially in women with long life expectancies.