1. Organized chronic cardiac thrombus from atrial fibrillation
2. Subacute thrombus from ischemia endocardium of acute MI
3. Iatrogenic catheter-induced thrombus
4. Paradoxical embolism from DVT, multiple PE with patent foramen ovale
5. Peripheral artery aneurysm – congenital or atherosclerotic origins
6. Atherosclerotic aortic plaque
7. Aortic aneurysm
Peripheral arterial aneurysms are an uncommon but important source of distal emboli. At the time of presentation, there is usually evidence of multiple prior episodes of small emboli followed by the current complete thrombosis and acute distal limb ischemia [15]. These aneurysms can be of both atherosclerotic and, less commonly, a congenital origin. Popliteal artery atherosclerotic aneurysms are the most common peripheral aneurysms and are associated with bilateral lesions in 50 % and abdominal aortic aneurysms in 60 % of patients [16]. Congenital popliteal entrapment and thoracic outlet syndrome with arterial compression can lead to aneurysms with embolic and thrombotic complications [17, 18]. Rupture of peripheral aneurysms is rare [16]. If left untreated, these rare congenital aneurysms are associated with a significant risk of limb loss because of chronic small distal emboli, acute thrombosis, and delay in diagnosis [17, 18].
The increasing use of the extremity arteries for diagnostic and therapeutic endovascular techniques had resulted in a rise in iatrogenic acute limb ischemia [9, 19, 20]. Catheterization site arterial occlusion may result from an intimal dissection, accumulation of thrombus along indwelling catheter sheaths with subsequent thrombosis or distal embolism, and intravascular occlusion from misplaced closure devices. Diagnosis of this source of acute ischemia is also frequently delayed. Similarly, the outcome is dependent upon prompt recognition and treatment.
The ischemic pattern with the classic “five P’s” of arterial occlusion occurs one level distal to the area of occlusion [5, 12]. If complete, this ischemia results in skeletal muscle and nerve tissue death at approximately 6 h [12]. This has given rise to the concept of a “Golden Period” of 6 h. This is the period of time between the onset of ischemia and the successful restoration of flow to save the limb from permanent loss of muscle and nerve tissue (Table 18.2).
Table 18.2
Acute and chronic signs and symptoms of extremity arterial occlusive disease
Acute ischemia |
Sudden onset of the “5 P’s” |
Pain |
Pulselessness |
Pallor |
Paresthesias |
Paralysis |
Absent or monophasic Doppler tones at the ankle or wrist |
Chronic arterial occlusion |
Mild |
Diminished distal pulses |
Mild claudication of legs or exercise-induced muscle pain arm |
Relieved by rest |
Ankle or wrist pressure index 0.6–0.75 |
Moderate |
Absent distal pulses |
Severe exercise-induced extremity pain |
Extremity pressure index < 0.6 |
Severe |
Night pain forefoot or numbness of hand |
No exercise tolerance |
Extremity pressure index < 0.5 |
Limb threat |
Rest pain in the extremity |
Nonhealing ulcers on digits, heel, or palm |
Dependent rubor |
Blanching on elevation |
Absent or monophasic distal Doppler tones |
Iatrogenic arterial occlusion is an increasingly common cause of acute limb ischemia [9]. The use of the femoral artery for catheter access for endovascular cardiac and peripheral interventions places a large number of patients at risk for acute ischemia from access site occlusions [19, 20]. Orthopedic surgical procedures in the hip and knee also put the femoral artery and the popliteal artery at risk for acute occlusion [22].
Acute massive venous thrombosis with outflow occlusion can cause acute limb ischemia. This entity is known as phlegmasia cerulea dolens or painful blue edema [23]. The venous obstruction and engorgement with desaturated blood is associated with significant pain and resulting in arterial vasoconstriction. Left untreated, limb-threatening ischemia results from the accumulation of desaturated venous blood, decreased arterial flow, and compartment syndrome [23].
Clinical Presentation and Diagnosis
Acute limb ischemia presents with sudden onset of pain followed quickly by numbness and weakness. In patients with preexisting occlusive disease, these symptoms may be less distinct [5, 12]. The most common clinical setting of acute limb ischemia includes a history of atrial fibrillation [5, 15]. Preexisting lower extremity occlusive arterial disease is the next most common setting. These acute on chronic limb ischemia patients will have a history of claudication past extremity angioplasty with stent placement or arterial bypass surgery. The third most common clinical setting is a history of either acute or chronic ischemic heart disease with thromboembolism when mural thrombus dislodges form the damaged endocardium [15]. There is rising incidence of catheterization site occlusion in patients undergoing cardiac endovascular procedures [9, 19]. These iatrogenic lesions also occur in the setting of associated cardiac disease.
History and physical examination quickly reveal the most likely source of the acute occlusion in the vast majority of patients. Cardiac source emboli usually occur in the setting of atrial fibrillation or acute myocardial infarction. Less commonly, emboli result from paradoxical embolism of venous thrombus through a patent foramen ovale in patients with lower extremity deep venous thrombosis and multiple pulmonary emboli [14]. The resulting pulmonary hypertension with opening of an incompletely closed foramen ovale allows the next venous embolism to cross from the right atrium through the foramen ovale into the left atrium and out to the systemic circulation. Rarely, emboli from atherosclerotic arterial plaque or a peripheral aneurysm present as acute limb ischemia [8, 15]. These lesions more commonly cause digital ischemia. Thrombosis of a preexisting bypass graft or a stent is an important source of acute ischemia in patients with previous surgical or endovascular management of chronic disease [13]. This group of patients usually has a progression of distal occlusive disease and less commonly, occlusion of a patent graft in the absence of identifiable progression of disease. These patients must undergo detailed catheter arteriography and often are candidates for thrombolytic therapy [13]. Consultation with a vascular surgeon colleague is usually required in this setting.
Most patients with acute limb ischemia from embolism do not have a history of claudication and have normal pulses in the contralateral, non-affected extremity [5, 8, 15]. Saddle embolism to the aortic bifurcation may result in an absence of palpable pulses in both legs [24]. Acute thrombosis of preexisting arterial occlusive disease usually occurs in the setting of preexisting claudication. Physical exam usually reveals diminished pulses in the contralateral extremity and signs of chronic ischemia in the affected extremity. Ankle brachial indices may also reveal contralateral occlusive disease (Table 18.2).
Complete thrombosis of the infrarenal aorta is a rare and usually catastrophic event [25]. Practically all of these patients have significant cardiac disease with poor cardiac output that leads to thrombosis of chronic occlusive disease of the distal aorta and iliac bifurcation. The clinical presentation may include buttock and leg muscle weakness or paraplegia from distal spinal cord and lumbar plexus ischemia. The diagnosis is often delayed because of the baffling constellation of clinical findings despite the fact that physical examination reveals the bilateral absence of pulses from the femoral arteries distally with lower body and leg mottling. The mortality rate in these patients exceeds 50 % [25].
Phlegmasia cerulea dolens presents in the setting of massive lower extremity DVT complicated by arterial vasoconstriction and dehydration [23]. This lower extremity and, rarely, upper extremity syndrome is striking in its appearance with a swollen, blue, and cool to the touch limb [23]. Severe pain is always present. The compelling nature of these findings usually prompts venous duplex studies which confirm the diagnosis.
Doppler assessment of arterial flow in the extremity is an essential adjunctive measure to add to physical examination. Although the experienced examiner can assess flow based on the character of the audible Doppler signals, the best way to use the Doppler device is in conjunction with an extremity systolic blood pressure determination. The manual blood pressure cuff is placed at the wrist or ankle and the probe placed over the distal vessel. The cuff is slowly inflated and the cessation of signals indicates the systolic blood pressure at the level of the cuff. Normal ankle brachial index (ABI) is 1.1 [6]. Less than 0.8 is abnormal. In general claudication begins to be significant at that level of reduced flow. Below an ABI 0.5 or an ankle systolic pressure of 60 Torr, potentially limb-threatening ischemia begins [6] (Table 18.2).
Once the diagnosis of acute limb ischemia due to cardiac source embolism is made, there is an important decision to carefully consider. If the patient with distal limb ischemia has atrial fibrillation, no history of prior claudication or arm exercise intolerance, and an otherwise normal peripheral vascular examination with a normal groin or axillary pulse proximal to the ischemic area, and normal contralateral limb pulses, immediate operative exploration for embolectomy is indicated [8]. Preoperative CT or catheter arteriography is essential in patients who do not have a clearly identifiable cardiac source embolism or an obvious level of arterial occlusion. Acute on chronic occlusion needs to be delineated with detailed arteriography [12]. Although CT angiography may be adequate, catheter arteriography is preferred. In the setting of preexisting chronic occlusive disease, catheter arteriography also allows for endovascular techniques when appropriate [26, 27].
The most important factor to consider in obtaining arteriography is the time it takes and the potential delay to the operating room. Remembering the “Golden Period,” there must be a rapid workup if arterial flow is to be successfully restored within 6 h. Therefore, if endovascular therapy is not an option, high-resolution CT angiography may be the best option if catheter arteriography is not immediately available.
Compartment syndrome should be anticipated in patients with acute limb ischemia [28]. All muscle compartments in the extremities are vulnerable to reperfusion intra-compartmental hypertension that can lead to muscle necrosis after revascularization [49]. Compartment syndrome results from post-ischemia swelling of muscle in the confined space created by the muscle fascia in the extremities. This swelling increases the tissue pressure within the compartment compressing venous and lymphatic outflow as well as arteriolar inflow [28]. Ultimately the tissue perfusion pressure threshold of 25 mmHg is exceeded and ischemic neurolysis and myonecrosis occurs. It may take hours to occur after restoration of flow and therefore serial examinations are essential to prevent delaying diagnosis and treatment. Compartment pressures should be measured with the Stryker device or by other devices (Fig. 18.1). Compartment syndrome occurs most commonly in the muscle compartments of the calf and is relatively uncommon in nontraumatic arterial occlusion in the upper extremity [28].
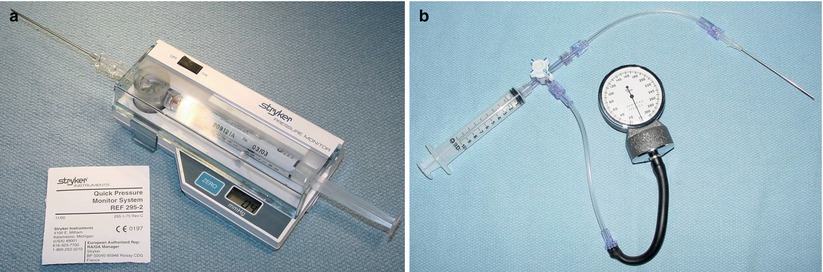

Fig. 18.1
(a) Stryker™ pressure monitoring device for muscle compartment pressure measurement. (b) Alternative pressure monitoring device for muscle compartment pressure measurement. The syringe and half of the line to the manometer is filled with saline (Reproduced, with permission, from Mattox KL, Moore EE, Fleiciano DV, eds.TRAUMA: New York: McGraw Hill; 2013)
Operative Management
The performance of emergency vascular surgery should be limited to those surgeons who are capable and qualified. That does not limit these procedures to those who are board certified in vascular surgery. There are many general surgeons who are very skilled in vascular technique by virtue of their interest and experience. There is a simple test to consider when deciding who should manage vascular emergencies. “Would I be comfortable having someone with this surgeon’s level of experience caring for a member of my family?” There should be a designated call panel for appropriate vascular surgical backup at all times for an acute care surgery service.
The use of checklists to manage acute surgical emergencies is strongly recommended. These are best prepared in advance and should be printed and posted in the operating room. In my practice, I have created short checklists for each of the most common vascular emergencies. I print three copies just before the operation and give one to the anesthesiologist, one to be posted on the operating room white board, one to hang on an i.v. pole at the head of the table within reading distance of the surgeons. If checklists haven’t been created, I recommend writing the important goals of the operation on the white board for all to see and to remind the team of the intended operative plan. Examples of checklists are provided below and I recommend each surgeon create their own version to include their choices for operative management (Tables 18.3 and 18.4).
Table 18.3
Checklist for femoral thrombectomy for cardiac source embolism and ischemic leg
1. Pull up A-grams on PACS, position patient, pads under ankles, time out, (start dextran 40), give antibiotics, choose sutures |
2. Decide on early fasciotomy |
3. Mark landmarks and sketch incision sites on leg |
3. Expose vessels and control with vessel loops |
4. Administer heparin bolus 5,000 units |
5. Choose transverse vs. longitudinal arteriotomy |
6. Fogarty catheter thrombectomy proximal and distal vessels |
7. Flush with heparinized saline |
8. Supplemental dose of 2,500 units of heparin at 50 min |
9. Close artery (PTFE patch if longitudinal arteriotomy) |
10. Pulse, Doppler interrogation, on-table agram as indicated |
11. If distal occlusion, go to distal popliteal via medial upper calf incision – repeat steps 5 and 6 |
12. Protamine, hemostasis, close wound, reassess pulses, Doppler if needed |
13. Reassess calf muscle compartments, measure pressures, fasciotomy if needed |
14. Talk to family, referring MD, and dictate |
Table 18.4
Checklist for 4-compartment calf fasciotomy
Calf fasciotomy checklist |
|---|
1. Mark landmarks and incision lines – note head of fibula and mark 2 finger breadths below for exclusion zone for peroneal nerve safety |
2. Skin incisions – full length medial and lateral to create full dermotomy |
3. Incise anterior compartment fascia through lateral incision proximally and distally, avoiding peroneal nerve exclusion zone proximally |
4. Probe under fascia to the tibia to assure in the anterior compartment |
5. Release lateral compartment fascia in similar fashion probing under fascia to confirm in proper space posterior to the intermuscular septum |
6. Posterior compartment release – generous longitudinal medial incision 2 cm behind tibia and avoid the saphenous vein |
7. Deep posterior release under direct vision to locate and avoid posterior tibial artery |
8. Hemostasis on skin, check muscle contraction with electrocautery in all compartments |
9. Place loose moist sponge or Kerlix in wounds – wrap leg loosely |
10. Recheck perfusion at DP, PT, dictate and complete chart work |
11. Reassess wounds for hemorrhage and dressing tension every 4–6 h for the next 24 h – avoid recurrent compression from dressings as muscle compartments swell after release |
The patient should be widely prepped and draped with generous inclusion of the entire upper or lower extremity and the shoulder or lower abdomen. One leg should also be prepped and draped from inguinal region to toes to allow for saphenous vein harvest. Adjunctive measures such as bolus intravenous systemic heparinization, the administration of a continuous infusion of low molecular weight DextranTM, and administration of intravenous antibiotics should be considered and utilized where appropriate. Preparation for surgery should also include appropriate management of associated cardiopulmonary disease by your anesthesia colleague.
Surgical exposure requires generous incisions placed to both maximize exposure and provide appropriate options for exploration and reconstruction. In the upper extremity, the axillary artery is exposed by making a transverse infraclavicular incision over the deltopectoral groove. A muscle-splitting incision is carried down through the pectoralis major muscle. The pectoralis minor muscle is divided close to the coracoid process and the axillary artery and vein exposed where they traverse just below the plane of the muscle. There are cords of the brachial plexus, nerves to the pectoral muscles, and large muscular branches of the artery in this area. Proximal and distal exposure of the artery should be carefully obtained avoiding damage to the brachial plexus and the axillary vein. Silastic vessel loops should be double passed proximally and distally and used to gently occlude the vessel. The artery is relatively fragile and pulling too vigorously on the vessel loops may fracture the arterial intima causing a dissection. A transverse arteriotomy is performed and proximal and distal thrombectomy with appropriately sized Fogarty catheters is carried out. A gentle heparinized saline flush (10 units heparin per ml) proximally and distally is performed taking care to not flush air or residual thrombus back up the vertebral arteries or the common carotid artery on the right side which can cause cerebral emboli and stroke.
The brachial artery is best exposed through a longitudinal incision along the medial aspect of the upper arm over the groove between the triceps and biceps muscles. The incision can be extended distally with an “S”-shaped extension across the antecubital fossa from ulnar to radial aspect and onto the forearm to expose the origins of the forearm vessels when the occluding thrombus is located at that level. (See below in Case Presentation 1.)
The location of incisions for acute lower extremity ischemia is determined by the level of the embolic occlusion. If the acute ischemic episode is due to a thrombosis of severe preexisting occlusive disease, a bypass graft, or a stented arterial segment, a vascular surgery colleague should be consulted to appropriately manage this complex problem. Iliac or femoral artery occlusion from an embolism is best approached through a longitudinal incision over the common femoral artery in the groin. The common, superficial, and profunda femoral arteries are controlled with silastic vessel loops. If the common femoral is soft and free of significant atherosclerotic plaque, a transverse arteriotomy is made. If it is not and chronic atherosclerotic changes are present, a longitudinal arteriotomy is the safest approach. Proximal and distal Fogarty catheter thrombectomy is carried out followed by gentle flushing with heparinized saline. The transverse arteriotomy is closed primarily with a running Prolene™ suture. A longitudinal arteriotomy should be closed with a patch angioplasty. Distal flow is assessed by pulse and Doppler examination or, if needed, completion intraoperative arteriogram. If distal thrombus is present and needs to be removed, a medial longitudinal incision along the posterior aspect of the tibia below the knee provides access to the distal popliteal artery. The exposure of the proximal tibial vessels may be required for distal control and should be carefully performed to avoid injury to the popliteal vein and the tibial nerve (Fig. 18.2). A checklist for management of lower extremity acute ischemia due to a cardiac source embolism is helpful for even the most experienced surgeon (Table 18.3).
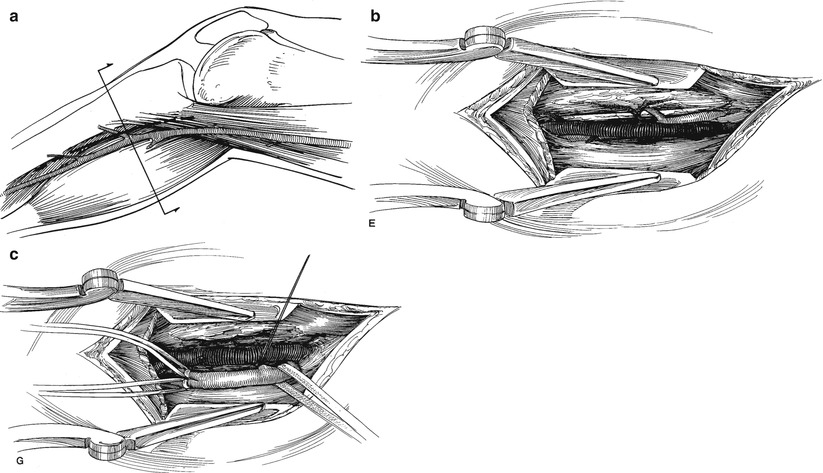

Fig. 18.2
(a) Incision for exposure vessels in the upper calf. (b) Dissection to expose distal popliteal artery. (c) Dissection to expose origins of tibial vessels (Reproduced, with permission, from Rutherford RB, ed. Atlas of Vascular Surgery: Basic Techniques and Exposures. Phoadelphia: WB Saunders; 1993. © Elsevier)
The treatment of phlegmasia cerulea dolens is based on prompt anticoagulation and catheter-directed thrombolytic therapy [23]. Pain management and hydration are also important. An inferior vena cava filter should be placed at the initiation of thrombolysis because of the risk of pulmonary embolism [30]. Open thrombectomy of the common femoral and iliac vein is very infrequently required [30]. At the time of opening the common femoral vein, careful use of a large Fogarty catheter in the iliac vein retrieves distal thrombus. Wrapping the leg firmly with a sterile elastic bandage will milk out proximal thrombus. Retrograde Fogarty catheter passage down the veins of the leg risks significant damage to the valves and worsens the risk of recurrent thrombosis.
Fasciotomy
Failure to perform an adequate fasciotomy when indicated after revascularization of an acutely ischemic limb is the most common cause of preventable limb loss [28, 29]. Calf compartment syndrome is common and forearm compartment syndrome is relatively rare in nontraumatic acute limb ischemia [28]. Calf fasciotomy, particularly in the setting of prolonged ischemia, must always be considered prior to completion of the operation. Intraoperative compartment pressure measurements may provide decision-making data [28, 29]. However, if normal pressures are initially obtained, eventual reperfusion edema and subsequent swelling may occur with delayed compartment syndrome. Serial postoperative compartment pressure measurements may be required. There are four compartments in the calf that need to be released. These include the anterior and lateral compartments on the anterolateral aspect of the calf and the deep and superficial posterior compartments (Fig. 18.3). The best approach for release is two long incisions: one on the lateral side and one on the medial side of the calf [31] (Fig. 18.4). Although isolated anterior compartment syndrome occurs in some settings, four compartment release is usually required. A checklist for fasciotomy is strongly recommended and an example is included in Table 18.4.
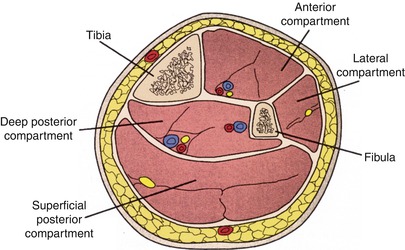
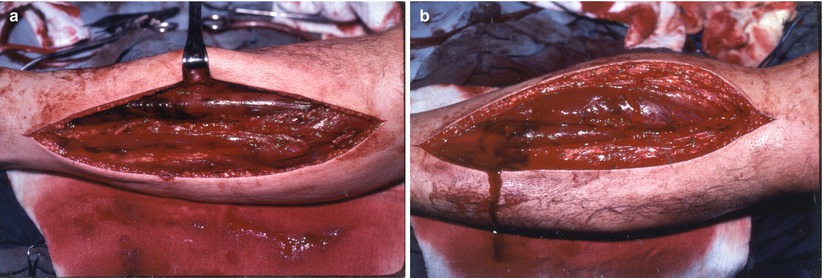

Fig. 18.3
Calf muscle compartments (Reproduced, with permission, from Frykberg ER: Compartment Syndrome, in Current Surgical Therapy, Cameron JD (ed) 5th edition, /WB Saunders, Philadelphia, 1995. © Elsevier)

Fig. 18.4
(a) Lateral incision for release anterior and lateral muscle compartments. (b) Medial incision for posterior and deep posterior compartments
The lateral calf incision should be generous. Start proximally no higher than 3–4 cm below the fibular head in order to avoid the superficial branch of the peroneal nerve. The incision should be taken distally to within 3–4 cm of the lateral malleolus. The fascia of both the anterior and the lateral compartments need to be incised longitudinally through the full length of the skin incision. Take care not to carry the incision beyond the limits of the skin incision proximally to avoid injuring the peroneal nerve. Make certain that the anterior compartment is fully released by palpating the tibia anteriorly under the fascia. Misplacing the incision lateral to the interosseous membrane will fail to decompress the anterior compartment with devastating consequences.
The medial incision should be made 1–2 cm posterior to the posterior edge of the tibia avoiding laceration of the greater saphenous vein. The fascia over the gastrocnemius should be fully incised proximally and distally. The gastrocnemius and soleus muscles are retracted posteriorly in the distal calf to expose the deep posterior fascia. This layer needs to be incised under direct vision to avoid lacerating the posterior tibial artery.
Once all four compartments are adequately released and adequate hemostasis is obtained, a loose dressing is applied. Care should be taken to avoid tight dressings which can recreate the compartment syndrome when muscle swelling occurs. Subsequent wound closure is performed in 2–3 days or when edema has sufficiently resolved. Split thickness skin graft may be required for closure.
Postoperative Considerations
Serial examinations after successful restoration of flow are essential to detect reocclusion or compartment syndrome and promptly treat these limb-threatening complications. These patients usually have significant comorbidities, and postoperative care should include a period of monitoring in the intensive care unit or a specialized telemetry unit. The nursing staff needs to have training and experience in monitoring the vascular status of extremities. Any evidence of recurrent ischemia, operative site hemorrhage, or compartment syndrome should prompt an immediate return to the operating room. In patients with cardiac source embolism, systemic anticoagulation should be started at 12 h or so after operation. The risk of recurrent embolism outweighs the risk of operative site bleeding. Continuous heparin infusion without a bolus is recommended instead of weight-based subcutaneous fractionated heparin. In the early postoperative period it is best to preserve the ability to reverse the intravenous infusion of heparin with protamine if needed because of bleeding complications. Once the patient is stable, the subcutaneous fractionated heparin every 12 h and oral warfarin can be administered. Lifelong anticoagulation is the standard of care in patients with a cardiac source embolism.
Acute Mesenteric Ischemia
Acute mesenteric ischemia is a potentially lethal process which requires prompt recognition and treatment for successful management. The mortality rate remains more than 50 % and there is little room for either delay or errors in management [32, 33]. Symptoms vary from the insidious onset of vague generalized abdominal pain to the sudden onset of severe and constant pain. There are four common causes: acute cardiac source embolism to the superior mesenteric artery, acute thrombosis of previous partial occlusion from an atherosclerotic lesion, splanchnic vasoconstriction leading to low flow and regional ischemia known also as nonocclusive mesenteric ischemia, and mesenteric venous thrombosis (Table 18.5). Each of these causes is a secondary phenomenon which results from other major diseases and occurs in a high-risk setting [34, 35] (Table 18.6).
Table 18.5
Etiology of mesenteric ischemia
50 % | Arterial embolism |
20 % | Arterial thrombosis |
20 % | Small vessel occlusion |
10 % | Venous thrombosis |
Table 18.6
Risk factors for mesenteric ischemia
Arterial embolism or thrombosis |
Cardiac disease: |
Atrial fibrillation |
Recent myocardial infarction |
Congestive heart failure |
Digitalis therapy |
Previous arterial emboli |
Hypercoagulable state |
Hypovolemia, shock |
Venous thrombosis |
Portal hypertension |
Intra-abdominal inflammation |
Trauma or major bowel surgery |
Prothrombotic state |
Chronic renal failure |
Pathophysiology
The arterial blood supply of the gut is divided into four areas defined by the arteries that supply them. Collaterals connect the perfusion of each area (Table 18.7) [36, 37, 39]. In the absence of preexisting occlusive disease and compensatory enlargement of collateral vessels, these connections are not sufficient to provide adequate flow if the superior mesenteric artery is acutely occluded. In chronic mesenteric occlusive disease from atherosclerosis, patients may have total gut perfusion via a single remaining mesenteric artery or the bilateral hypogastric arteries via collateral flow to the other vessels [38]. However, many of these patients have intestinal angina when eating large meals. The venous drainage of the gut is via the portal venous system. Gastric drainage is via the splenic vein. The small bowel and the proximal colon up to the splenic flexure drain via the superior mesenteric vein. The descending colon drains via the inferior mesenteric vein. Collateral venous vessels are also present and connect each major area.
Table 18.7
Gut regions, their blood supply, and collateral connections
Region | Blood supply | Collateral connections |
|---|---|---|
Foregut | Celiac artery | Pancreaticoduodenal arteries and arc of Buhler distally |
Distal esophagus through the ampulla of Vater in the duodenum | ||
Midgut | Superior mesenteric artery | Pancreaticoduodenal arteries and arc of Buhler proximally Marginal artery of Drummond and arc of Riolan distally |
Ampulla of Vater region of the duodenum to splenic flexure of the colon | ||
Hindgut | Inferior mesenteric artery | Marginal artery of Drummond and arc of Riolan proximally |
Splenic flexure of the colon to distal sigmoid colon | Superior hemorrhoidal to middle hemorrhoidal arteries distally | |
Cloacal derivatives | Branches of the bilateral hypogastric arteries | Middle hemorrhoidal to superior hemorrhoidal arteries proximally |
Cardiac source emboli have a predilection to enter the orifice of the relatively large superior mesenteric artery and then typically lodge distal to the origin of proximal jejunal branches and the middle colic artery [38]. This gives rise to a pattern of small intestine and colon ischemia with sparing of the proximal jejunum and perfusion of the transverse colon and distal colon. Celiac artery emboli are less common as are emboli to the inferior mesenteric artery and hypogastric artery emboli rarely cause ischemia due to a variety of pelvic collateral arteries [38].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree