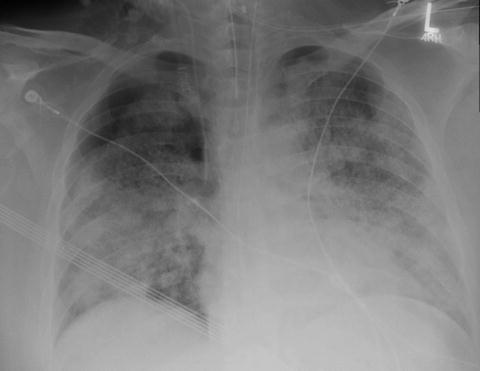
Fig. 7.1
Timing of pulmonary complications days after HSCT
Mucormycosis is typically indistinguishable from invasive aspergillosis, but more frequently has a “reverse halo sign,” a round area of ground glass opacities surrounded by a crescent or complete ring of consolidation [17]. Pulmonary mucormycosis has a high mortality, often as high as 80 % [18]. Early recognition and treatment with lipid amphotericin B formulations and/or posaconazole can improve outcomes [19]. Patients with diabetes mellitus are predisposed to be infected with mucormycosis compared to those who do not have diabetes [20]. One study of infections in hematologic patients found that 56 % of the patients with mucormycosis were diabetic [20].
Following HSCT, patients are typically placed on prophylactic trimethoprim/sulfamethoxazole to reduce infection from Pneumocystis jiroveci. Thirty to hundred days after HSCT, patients are frequently found to have Pneumocystis jiroveci induced pneumonia. In a study over 10 years at a single center, the diagnosis of Pneumocystis jiroveci was confirmed in a total of 154 patients negative for HIV [21]. The most common comorbid condition was a hematologic malignancy in 32.5 % of the cases [21]. Diffuse alveolar or interstitial opacities are often observed on plain radiographs [22]. On CT, a common pattern is widespread perihilar ground-glass opacities in a mosaic pattern, reflecting interspersion of areas of infected lung tissue with normal lung parenchyma [6].
Diagnosis of Pneumocystis jiroveci is most commonly obtained by polymerase chain reaction (PCR) of bronchoalveolar lavage fluid, fungal culture of bronchoalveolar lavage fluid, or transbronchial biopsy (TBLB) of infected lung tissue. Elevated LDH greater than 220 and beta-D-glucan serum testing can indicate likelihood of infection. PCR has a sensitivity of 72–100 % and specificity of 86.2 % in studies [23]. Bronchoscopic evaluation and subsequent evaluation of BAL represents the best approach to the identification of Pneumocystis jiroveci. Kim et al. demonstrated an incidence of Pneumocystis jiroveci in 17 % in patients who had a hematologic malignancy that underwent bronchoscopy with BAL and subsequent analysis with specific immunofluorescence staining and real time PCR [4].
Pneumonia from Candida spp. appears on imaging as any of the following: multiple patches of consolidation, cavitation and pulmonary nodules [9]. Empiric echinocandin coverage with narrowing of antibiotics after speciation is appropriate treatment. Similar to pneumonia caused by P jiroveci, pneumonia from Candida is seen less frequently with appropriate prophylactic treatment of patients with hematologic malignancies.
Viral Infections
With the utilization of molecular based diagnostic tools for viral pathogens, patients with hematologic malignancies that present with respiratory symptoms are increasingly screened for viral etiologies. CMV is the most common cause of viral pneumonia, yet morbidity is also caused by rhinoviruses, adenoviruses, influenza viruses, parainfluenza viruses, respiratory syncytial virus, and metapneumovirus [8]. Chest imaging of patients with viral pneumonia have similar appearances regardless of pathogen [24].
CMV pneumonia usually occurs in patients 31–100 days after HSCT. The most common radiographic findings are bilaterally distributed parenchymal opacities and innumerable nodules [8]. Prophylaxis with valganciclovir is the mainstay for patients after HSCT. Mortality rates of CMV pneumonia in patients after hematopoietic cell transplants are 50 %, but increase to 85 % if left untreated [25, 26]. Ganciclovir is the primary treatment for CMV pneumonia. CMV serum titers are often utilized to guide detection and management. The diagnosis can be made by analysis of the BAL fluid by specific immunofluorescence staining and real time PCR [4]. Identification of CMV is made by transbronchial lung biopsy or bronchoalveolar lavage (BAL) fluid (in the proper clinical setting) by a sensitive immunocytochemical method [27].
Noninfectious Acute Pulmonary Manifestations
The noninfectious pulmonary complications of hematologic malignancies carry significant morbidity and mortality as do the infectious complications. Noninfectious complications such as pulmonary hemorrhage and pulmonary edema can arise in any of the hematologic malignancies. Pulmonary leukostasis occurs in acute leukemia, most commonly in the myelomonocytic subtypes, and is a result of the disease process itself. Alternatively, there are therapies used in treatment of specific hematologic malignancies that can cause pulmonary complications. Hematopoietic stem cell transplantation (HSCT) can lead to infectious complications as mentioned above, but also noninfectious issues arise that affect the pulmonary system. Pulmonary complications can lead to life threatening illness in a patient with a hematologic malignancy. The specific associations with therapies should be well known to physicians treating these patients. Prompt recognition of the pulmonary complication and initiation of treatment is paramount for this patient population (Table 7.1).
Table 7.1
Infectious acute pulmonary manifestations of acute hematologic malignancies
Pulmonary Infection | Clinical setting | Cause of immunosuppression | Common pathogens | Radiologic findings | Evaluation | Treatment |
|---|---|---|---|---|---|---|
Bacterial pneumonia | Neutropenia | Leukemia, myelodysplastic syndrome, chemotherapy, recent (<30 days) HSCT | Gram-negative bacilli, gram- positive cocci | Segmental or lobar consolidation | Blood culture, sputum sample, analysis of BAL | Broad spectrum antibiotics, anti-pseudomonal, narrow on culture data |
T cell defect | Lymphomas, lymphoblastic leukemia, chemotherapy, monoclonal antibodies, corticosteroids, HSCT 30–100 days | Nocardia, Mycobacterium | Cavitation, lobar consolidation, nodules | AFB smears and culture, analysis of BAL | Nocardia Bactrim Mycobacterium pending speciation | |
B cell defect | Lymphoma, leukemia, multiple myeloma, splenectomy | Encapsulated bacteria (Streptococcus pneumonia, H. influenza) | Segmental or lobar consolidation | Blood culture, sputum sample, analysis of BAL | Broad spectrum antibiotics, anti-Pseudomonal, narrow on culture data | |
Viral pneumonia | T cell defect | Lymphomas, lymphoblastic leukemia, chemotherapy, monoclonal antibodies, corticosteroids, HSCT 30–100 days | CMV Multiple other viruses | Ground-glass opacities, micronodules, airspace consolidation | Detection of CMV in BAL fluid or lung tissue samples, CMV serum PCR | CMV: Ganciclovir Supportive care |
Fungal pneumonia | Neutropenia | Leukemia, myelodysplastic syndrome, chemotherapy, recent (<30 days) HSCT | Candida, Aspergillus | Aspergillus: “halo” sign, segmental or subsegmental pleura-based consolidation Candida: multiple microabscesses | Aspergillus: Galactomannan antigen test, analysis of BAL Candida: blood culture, analysis of BAL | Echinocandins in severe infections, narrow to Itraconazole or Voriconazole |
T cell defect | Lymphomas, lymphoblastic leukemia, chemotherapy, monoclonal antibodies, corticosteroids, HSCT 30–100 days | Pneumocystis jiroveci Aspergillus (as above) | Widespread perihilar ground-glass opacities | LDH, Detection of P jiroveci in BAL analysis, PCR | Bactrim |
Pulmonary Hemorrhage
Pulmonary hemorrhage is the most common noninfectious pulmonary complication of acute leukemia [28]. Pulmonary hemorrhage is associated with thrombocytopenia, infectious disease, and post HSCT. Diffuse alveolar hemorrhage is observed in approximately 20 % of patients who have undergone HSCT and typically manifests within the first few weeks post-transplantation [8] (Table 7.2).
Table 7.2
Noninfectious acute pulmonary manifestations of patients with hematologic malignancies
Complication | Clinical setting | Radiologic findings | Evaluation | Treatment |
|---|---|---|---|---|
Pulmonary hemorrhage | Thrombocytopenia, sudden onset of respiratory symptoms, rarely hemoptysis | Rapid progression of diffuse ground glass opacities and/or consolidation | Bronchoscopy, analysis of BAL | Augmentation with blood products, corticosteroids |
Pulmonary edema | Multiple transfusions, cardiotoxic chemotherapy, renal impairment, large volume of intravenous fluids | Redistribution of blood flow toward the upper lobes, increased interstitial markings | Imaging, weights, strict measurement of intake and output | Fluid restriction (when able), diuretics |
Leukostasis | Hyperleukocytosis (WBC > 100,000) and acute myelomonocytic leukemia (AML subtype M4) | Interstitial and/or alveolar opacities in varying degrees | Laboratory data, imaging, bone marrow bx | Leukapheresis, reduction in WBC |
Hemoptysis is an uncommon initial presentation in patients with hematologic malignancy that have developed pulmonary hemorrhage. Rather, the clinical presentation consists typically of the sudden onset of progressive dyspnea, nonproductive cough, fever and hypoxia. Radiographic findings may appear to be out-of-proportion to the patient’s relatively mild symptoms [29]. The radiographic changes progress rapidly to diffuse ground-glass opacities and patchy consolidation (Fig. 7.2) [29]. CT findings include widespread ground glass opacities, consolidation and reticulation or a crazy-paving pattern [30].
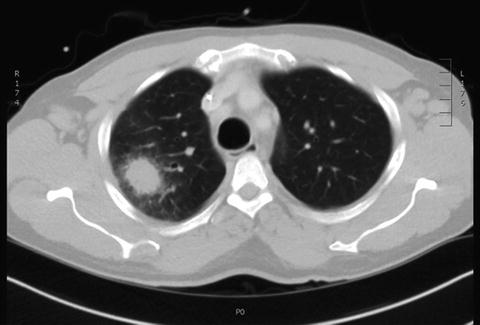

Fig. 7.2
Computed tomography slice demonstrating the classic halo sign associated with aspergillus infection in a 44-yo male with neutropenia. Image courtesy of Mark King, MD. Division Chief Thoracic Imaging. The Ohio State University. Columbus, OH
Bronchoscopic evaluation of the airway establishes the diagnosis of pulmonary hemorrhage. The BAL will demonstrate abnormally increased blood content that increases with subsequent aliquots of lavage. Microscopic evaluation of the BAL will show macrophages with hemosiderin content greater than 20 % without evidence of infection [31]. Importantly, infection can be a cause of pulmonary hemorrhage or hemoptysis. Therefore, careful examination of the BAL fluid for infectious organisms must be performed.
Treatment of pulmonary hemorrhage includes correcting coagulopathy, augmenting platelet counts with transfusion and supporting the patient during respiratory compromise.
Pulmonary Edema
Patients with hematologic malignancies require intravenous administration of many medications including renal protective fluids during chemotherapy infusions. With the increased amount of volume introduced to the patient, pulmonary edema can develop. The etiology is multifactorial and includes increased hydrostatic pressure from high-volume infusions, multiple transfusions, parenteral nutrition, cardiotoxic effects of chemotherapy, renal impairment, and increased permeability of pulmonary vessels caused by a variety of other factors [32].
Radiographic evidence of pulmonary edema often includes cardiomegaly, redistribution of blood flow toward the upper lobes (cephalization), increased interstitial markings, Kerley B lines, and perihilar or peribronchial areas of ground glass opacities [8]. On CT, the same findings can indicate pulmonary edema with the addition of interlobular septal thickening [32].
Volume sparing strategies and diuresis are the mainstay of treatment for pulmonary edema. Supportive care for respiratory failure with noninvasive positive pressure ventilation can help reduce intubation and invasive mechanical ventilation. Early recognition and prompt treatment with diuretic agents improve outcomes.
Pulmonary Leukostasis
Patients with acute leukemia presenting with an initial hyperleukocytosis (white blood cell count of more than 100,000 per microliter) are at risk for pulmonary leukostasis. Leukemic cells accumulate in minor blood vessels in the lungs during pulmonary leukostasis. Other sites of accumulation include the heart, brain and testes. Patients typically present with cough, fever and dyspnea. In particular, patients with myelomonocytic subtypes of AML (M4/M5) are at increased risk that may be predicted by a specific surface marker [8]. The expression of CD56/NCAM, a surface marker used in routine immunophenotyping of AML, may help to predict severe and potentially fatal leukostasis in hyperleukocytic acute myelomonocytic leukemia [33]. Only the absolute count of CD56 positive blasts was a significant predictor of risk of severe leukostasis (P = 0.020) [33]. This was not observed in AML without monocytic involvement (AML M1, M2, M3v) [33].
Chest radiographs in pulmonary leukostasis vary from normal to degrees of interstitial or alveolar opacification [34]. Chest CT findings of thickening of the bronchovascular bundles and prominence of peripheral pulmonary arteries correlate with the histopathologic findings [8] (Fig. 7.3).