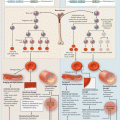
Fig. 8.1
Timeline of infectious and noninfectious pulmonary complications after allogeneic hematopoietic cell transplantation. AFOP acute fibrinous and organizing pneumonia, AIP acute interstitial pneumonitis, ARDS acute respiratory distress syndrome, BOS bronchiolitis obliterans syndrome, COP cryptogenic organizing pneumonia, CMV cytomegalovirus, GVHD graft-versus-host disease, HHV-6 human herpesvirus-6, HMPV human metapneumovirus, PAP pulmonary alveolar proteinosis, PCT pulmonary cytolytic thrombi, PIV parainfluenza virus, PVOD pulmonary veno-occlusive disease, PERDS peri-engraftment respiratory distress syndrome, VTE venous thromboembolism, RSV respiratory syncytial virus. *PAP has been reported to occur >1 year post-HCT. Adapted from Ref. [13]
These noninfectious etiologies must be distinguished from infectious complications in order to tailor antimicrobial therapy and to guide decisions to empirically augment immunosuppression. Computed tomography (CT) of the chest is mandatory in initial evaluation of respiratory failure, and radiographic patterns may suggest specific etiologies. The differential diagnosis of focal or diffuse infiltrates is broad, and further evaluation is usually undertaken.
Fiberoptic bronchoscopy with bronchoalveolar lavage (BAL) is a minimally invasive modality which is relied upon to make a specific diagnosis for new onset pulmonary infiltrates. The utility of bronchoscopy lies in exclusion of infection when clinical suspicion for a noninfectious etiology is high as much as it is to establish a specific infectious etiology [13]. The diagnostic yield of BAL has improved significantly for specific infectious diagnoses due to the advancements in diagnostic testing, including CMV rapid shell vial culture, aspergillus galactomannan, and respiratory viral PCR panel (Table 8.1) [14]. Two retrospective studies done in the era of routine anti-infective prophylaxis against fungus, CMV, and Pneumocystis jirovecii, report the yield of fiberoptic bronchoscopy for a specific diagnosis is 34–50 %, with a higher yield for allogeneic HCT [15, 16]. The majority of the diagnoses are achievable by BAL alone, with transbronchial biopsy adding additional specific information in <10 % of cases.
Table 8.1
Laboratory evaluation of bronchoalveolar lavage specimens for specific infectious agents
Bacterial | Fungal | Viral | Parasitic | |
|---|---|---|---|---|
Pathologic exam | Wright-Giemsa Papanicolaou’s Modified Jimenez stain (Legionella) | Silver stain (Pneumocystis jirovecii) Fungal hyphae Yeast forms | Viral inclusions (CMV) | Larval forms (Strongyloides) |
Microbiology—stains | Gram stain Acid fast Modified acid fast | Wet mount KOH Calcofluor white stain | ||
Culture | Aerobic and anaerobic Chocolate yeast extract (Legionella) Acid-fast Nocardia Actinomycoses | Fungal | Routine viral culture Rapid centrifugation “shell vial” (CMV, RSV) | |
Immunoassay | DFA (Legionella) | ELISA for galactomannan IFA/DFA (Pneumocystis jirovecii) | DFA (influenza) DFA (CMV) | Immunostain (Toxoplasma) |
Molecular diagnostics: PCR | Chlamydia Mycoplasma | Aspergillus Pan-fungal (including Mucorales spp. | Herpesviruses: CMV, EBV, HSV, VZV, HHV-6 CRVs: RSV, influenza, PIV-3, hMPV, rhinovirus, bocavirus, adenovirus Coronavirus | Toxoplasma |
While aggressive diagnostic workup is appropriate for HCT patients, the impact of BAL on patient outcomes is difficult to ascertain [13]. Various factors including the timing of procedure may influence the clinical outcome, as patients are often referred for BAL after persistence or progression of disease on empiric antibiotic therapy. A large retrospective study of 501 consecutive patients who underwent 598 BALs for the evaluation of new pulmonary infiltrates and suspected pneumonia during the first 100 days after HCT showed that the yield of BAL for clinically significant pathogens was 2.5-fold higher (p < 0.0001) if performed within the first 4 days of presentation, compared to those performed late, and that the yield was highest when performed within 24 h of presentation. Mortality at 30 and 100 days following the BAL was significantly lower when a diagnosis of infection was confirmed by early BAL compared to late BAL, which was associated with multidrug-resistant and polymicrobial infections [17]. These findings suggest that BAL should be used in the early evaluation of HCT recipients with pulmonary infiltrates, as opposed to a conservative, expectant approach of bronchoscopy only after the failure of empiric therapy.
The use of bronchoscopy has largely supplanted surgical biopsy as means of diagnosing unknown lung disease in HCT patients. When treatment decisions depend on a definitive diagnosis of a noninfectious etiology and when empiric therapy is risky, surgical lung biopsy may be required to achieve a specific diagnosis, as is that case for pulmonary venoocclusive disease and pulmonary cytologic thrombi, for example. Surgical lung biopsy has the advantage of direct visualization of the biopsy site, as well as obtaining a large enough piece of tissue that allows for histologic examination and culture. Bleeding in the setting of thrombocytopenia can be controlled directly. In contemporary practice surgical lung biopsy is usually performed videoscopically (video assisted thoracic surgery, or VATS), which theoretically reduces the morbidity associated with thoracotomy. Lung biopsy in this population must be carefully considered, as the morbidity of surgery in the setting of neutropenia, thrombocytopenia and/or respiratory failure may be unacceptably high, particularly if the overall prognosis is poor and specific therapy is unlikely to alter the outcome. White and colleagues at Memorial Sloan Kettering Cancer Center reviewed 67 open lung biopsies in 63 patients with hematologic malignancies from 1996 to 1998, including 25 bone marrow transplant patients, and found that a specific diagnosis was found in 41 (62 %) of the biopsies. Focal lesions were much more likely than diffuse lesions to yield a specific diagnosis (79 % vs. 36 %), and neutropenic or mechanically ventilated patients had a low likelihood of having a specific diagnosis. The risk of complications was significant, affecting 13 % of the biopsies, including 1 death [18].
In contrast to classic reports in bone marrow transplant recipients prior to the era of CMV and fungal prophylaxis [19, 20], infectious etiologies are no longer the most common diagnoses at lung biopsy: inflammatory conditions were the most common specific diagnoses (23 %), followed by infection (21 %) and malignancy (18 %) [18]. The likelihood of an HCT recipient undergoing a surgical lung biopsy has continued to decline in the past two decades, and infectious etiologies at lung biopsy, particularly invasive aspergillosis, are significantly less likely than a noninfectious etiology, likely due to the introduction of broad spectrum triazole therapy against invasive fungal infection in the early 2000s [21]. A recent meta-analysis showed that the overall yield of a specific diagnosis was similar between BAL and surgery (53 % vs. 54 %), with a complication rate of 8 and 15 % for BAL and surgery, respectively. The proportion of noninfectious diagnosis exceeded the infectious diagnoses with surgical biopsy [22].
Noninfectious Etiologies
Lung Injury Syndromes, Early Onset
Idiopathic Pneumonia Syndrome
A spectrum of acute lung injuries can develop in the early posttransplantation period that fall under the category of the idiopathic pneumonia syndrome (IPS), and includes diffuse alveolar hemorrhage, peri-engraftment syndrome, delayed pulmonary toxicity syndrome and acute fibrinous and organizing pneumonia (Table 8.2). Broadly defined as widespread alveolar injury seen after HCT in the absence of active lower respiratory tract infection, cardiac dysfunction, acute renal failure, or iatrogenic fluid overload, IPS is thought to result from a variety of lung insults including toxic effects of the HCT conditioning regimen, immunologic cell-mediated injury, inflammatory cytokines, and occult pulmonary infections [2]. With the increasing use of new diagnostic methods such as multiplex PCR for respiratory viruses and the biomarker galactomannan for Aspergillus spp., the incidence of acute lung injury secondary to pulmonary infection has increased. Seo et al. have shown that approximately half of patients with IPS had pathogens detected in the BAL when these newer diagnostic methods were employed and that pathogen detection was associated with increased mortality. The most frequent pathogens were human herpes virus-6 (HHV-6), human rhinovirus (HRV), cytomegalovirus (CMV), and Aspergillus spp. [23]. Of note, HHV-6, like CMV, is in the human β-herpesvirus family and latently infects 95 % of healthy adults. HHV-6 has been shown to reactivate frequently in critically ill patients, but its specific role in the pathogenesis of respiratory disease and outcomes in critical illness remains unknown [24].
Table 8.2
The spectrum of clinical lung injury entities after HCT classified under IPS
Clinical entity | Timing of onset after HCT | Histology | Diagnostic considerations | Prognosis |
|---|---|---|---|---|
Acute fibrinous organizing pneumonia (AFOP) | Early or late | Intra-alveolar fibrin, organizing pneumonia and type II pneumocyte hyperplasia | Poor | |
Acute interstitial pneumonia (AIP) | 2–6 mos | Diffuse alveolar damage | Poor | |
Acute respiratory distress syndrome (ARDS) | 2–6 mos | Diffuse alveolar damage | Poor | |
Cryptogenic organizing pneumonia (COP) | 2–12 mos | Patchy plugs of granulation tissue and macrophages in distal airways and alveoli | Chest CT: Ground glass & linear opacities, upper lobe predominance | Favorable |
Diffuse alveolar hemorrhage (DAH) | <1 mo–3 mos | Diffuse alveolar damage; intra-alveolar red blood cells and hemosiderin-laden macrophages | Progressively bloodier BAL fluid | Poor |
Delayed pulmonary toxicity syndrome (DPTS) | 1–6 mos | Type II pneumocyte hyperplasia, septal thickening, interstitial fibrosis, small vessel endothelial injury | Autologous transplants only | Favorable |
Peri-engraftment respiratory distress syndrome (PERDS) | Within 5–7 days of neutrophil engraftment | Diffuse alveolar damage | Neutrophilic inflammation on BAL | Favorable |
IPS has been classified into specific entities based in large part on the presumed site of primary tissue injury . The primary anatomical sites of inflammation and dysfunction are divided into three subtypes: (1) pulmonary parenchymal, (2) vascular endothelial, and (3) airway epithelial [25]. Each subtype is further classified into distinct entities of noninfectious acute lung injury. While some IPS cases remain unclassified, this classification scheme may facilitate the development of therapeutic interventions that can be directed toward distinct subtypes of disease [25] (Table 8.2).
An association between IPS and acute GVHD has been reported, but the specific role of alloreactive donor T lymphocytes in the pathogenesis of IPS remains unclear. Although identified in the lungs of some patients with IPS, epithelial apoptosis, considered pathognomonic for acute GVHD, has not been consistently detected in allogeneic HCT recipients with acute lung dysfunction. There is no pathognomonic histologic finding that defines IPS [25], as the histology depends upon the anatomic site of injury. The diagnosis of IPS is rarely confirmed by tissue biopsy given the significant risks of open lung biopsy in this population and the low yield of lung tissue by transbronchial biopsy via fiberoptic bronchoscopy recovered from HCT patients with acute lung injury. The diagnosis of IPS is usually one of exclusion in the appropriate clinical context; BAL is often performed to exclude infection.
The cumulative incidence of IPS after allogeneic HCT ranges from 2.2 % following nonmyeloablative conditioning to 8.4 % following conventional, full-intensity radiation containing preparative regimens [26]. The median time of onset after allogeneic HCT is 19 days (range, 4–106 days) with mortality rates ranging from 60 to 80 % overall to greater than 95 % for patients requiring mechanical ventilation. Although IPS also develops after autologous HCT, the incidence is lower, the median time to onset is generally later (63 days; range 7–336 days), the response to corticosteroids is usually prompt and the prognosis is favorable compared with IPS in allogeneic HCT recipients.
Risk factors for IPS after allogeneic HCT include full-intensity conditioning with total body irradiation, acute GVHD, older recipient age, an underlying diagnosis of acute leukemia or myelodysplastic syndrome and the number of platelet transfusions administered [27]. Risk factors for IPS following autologous HCT include older patient age, severe oral mucositis, conditioning regimens using total body irradiation or carmustine (BCNU), chest irradiation within 2 weeks before transplant, female gender, and an underlying diagnosis of solid tumor [2, 25].
Current standard treatment strategies of IPS include broad-spectrum antibiotics and IV corticosteroids and supportive care with lung protective mechanical ventilation and venovenous ultrafiltration for those with respiratory failure. Response to corticosteroids (≤2 mg/kg/day) has shown mixed efficacy in allogeneic HCT recipients, which likely reflects the diversity of underlying causes responsible for the lung insult. Compared with lower doses, higher doses of corticosteroid therapy (>2 mg/kg/day) have not been shown to improve outcome but are associated with increased iatrogenic complications including fungal infection [26, 28]. Prophylaxis against filamentous fungal infection with voriconazole or micafungin is recommended during treatment with corticosteroids (≥0.5 mg/kg/day) because fungal pneumonia was identified in 16 % (4/25) of IPS patients at the time of autopsy in a single-center study.
Preclinical and clinical studies suggest that neutralization of tumor necrosis factor (TNF)-α may be a useful therapeutic strategy for IPS. In a multicenter, Phase II single-arm, open-label study by Yanik and colleagues in pediatric HCT recipients, treatment with etanercept (0.4 mg/kg/dose twice weekly for 8 doses) plus corticosteroids (2 mg/kg/day) resulted in a complete response in 71 % (20 of 28) of patients and was associated with a high overall survival compared with historical controls [29]. In a randomized, double-blind, placebo-controlled trial of corticosteroids (2 mg/kg/day) with etanercept (0.4 mg/kg/dose twice weekly for 8 doses) or placebo for the treatment of IPS, the use of corticosteroids was associated with higher response rates (defined as alive with complete discontinuation of supplemental oxygen support at days 28 and 54 after initiation of therapy) and overall survival compared to historical controls, but the addition of etanercept did not lead to further increases in response [30]. This study was truncated early and the small sample size precluded a definitive conclusion regarding the benefit of etanercept therapy.
Diffuse alveolar hemorrhage (DAH ) , also called acute pulmonary hemorrhage or hemorrhagic alveolitis, is a common subset of IPS that generally develops in the immediate post-transplant period and is characterized by progressive dyspnea, cough, and hypoxemia with or without fever and diffuse bilateral pulmonary infiltrates (Fig. 8.2). The cumulative incidence of DAH is 5–12 % of HCT patients with a median time of onset of 19 days (range, 5–34 days) in allogeneic recipients and 12 days (range, 0–40 days) in autologous patients. The diagnosis is based on progressively bloodier return of BAL fluid. Treatment of DAH consists of aggressive platelet support to maintain a platelet count greater than or equal to 100,000 and high-dose systemic corticosteroids (2 mg/kg/day to 1 g/m2/day). The addition of aminocaproic acid or recombinant factor VII may further improve outcomes. Despite these interventions, the mortality from DAH ranges from 60 to 100 % with death usually due to multiorgan failure within 3 weeks of diagnosis [2].
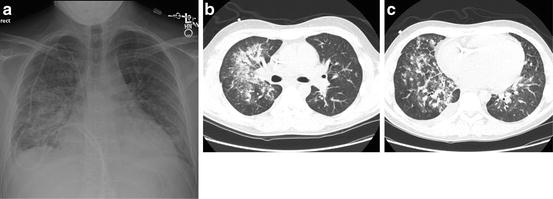

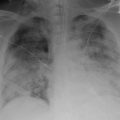
Fig. 8.2
DAH in a patient 23 days following an allogeneic stem cell transplant with new onset hemoptysis and respiratory insufficiency. DAH was diagnosed by bronchoscopy which showed progressively bloodier lavage fluid. (a) Chest radiograph shows patchy consolidation. (b, c) Chest CT shows diffuse ground glass opacities with areas of denser consolidation within a peribronchial vascular distribution
Peri–engraftment respiratory distress syndrome (PERDS) develops within 5–7 days of engraftment and accounts for 33 % of IPS cases after allogeneic HCT. Although the clinical presentation of PERDS after HCT is similar to other subsets of IPS, lung dysfunction is more responsive to corticosteroids and the prognosis is better [31].
The incidence of delayed pulmonary toxicity syndrome (DPTS) is 29–64 % in autologous HCT recipients receiving chemotherapy with regimens containing BCNU, cyclophosphamide, and cisplatin. The median time of onset is 45 days (range, 21–149 days), and treatment with corticosteroids (1 mg/kg/day) results in resolution in up to 92 % of cases [32].
Acute fibrinous and organizing pneumonia (AFOP) is an infrequently observed subset of IPS that is histologically characterized by the presence of intra-alveolar fibrin that form fibrin “balls” within the alveolar spaces, organizing pneumonia composed of intraluminal loose connective tissue within alveolar ducts and bronchioles in association with the fibrin and type II pneumocyte hyperplasia, typically in a patchy distribution. AFOP has been associated with a variety of disease states, including HCT [33], infections, rheumatologic diseases as well as environmental and drug exposures. The presenting symptoms may include fever, cough, dyspnea, hemoptysis, malaise, arthralgias, and chest, pleural, or abdominal pain. Two distinct clinical courses have been reported: (1) an acute and fulminant course typically leading to respiratory failure and death and (2) a less severe, subacute course. The radiographic findings of patients with an acute presentation and rapid decline are similar to those of diffuse alveolar damage including diffuse but basilar predominant consolidation and ground glass opacities. The radiographic features of patients with subacute AFOP are indistinguishable from those of organizing pneumonia with both focal and diffuse parenchymal abnormalities. Although no specific therapy exists, case reports describe response to corticosteroids [34] and immunosuppressant therapy [35].
Lung Injury Syndromes, Late Onset
Cryptogenic Organizing Pneumonia
Cryptogenic organizing pneumonia (COP) , originally given the term bronchiolitis obliterans organizing pneumonia (BOOP), was first described by Epler in 1985 as a distinct clinicopathologic entity that can affect the general population. As a histologic entity, organizing pneumonia (OP) may be associated with bacterial and viral infections, but most OP in the general population is idiopathic, hence the term cryptogenic organizing pneumonia (COP). In HCT recipients, the histology is characterized by patchy plugs of granulation tissue occupying distal terminal airways, alveolar ducts and alveolar sacs, widened alveolar septa and mononuclear cell infiltration, and accumulation of foamy macrophages within alveoli (Fig. 8.3) [36]. In the context of noninfectious pulmonary entities after HCT, COP may be considered a late-onset manifestation on one end of the spectrum of lung injury syndromes, of which IPS occupies the early onset, acute end of the spectrum [37].
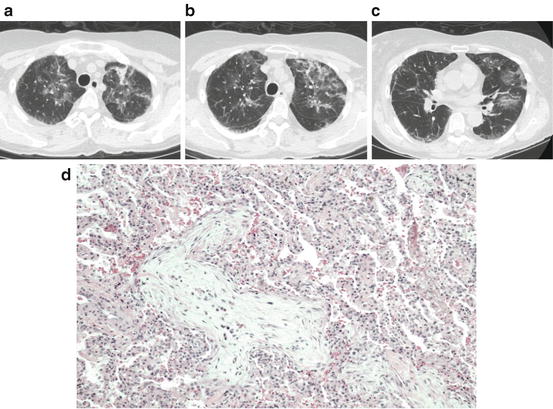

Fig. 8.3
COP after HCT. (a–c) Serial Chest CT images in the same individual showing upper lobe predominant reticular linear and ground glass opacities. (d) Example of COP on lung biopsy showing patchy plugs of granulation tissue within distal small airways and alveolar spaces with macrophages and inflammatory infiltrate. Courtesy of Drs. Robert Hackman and David Myerson
In an analysis of 49 biopsy-proven cases of COP after allogeneic HCT, there was a significant association with the presence of acute or chronic graft-versus-host disease (GVHD) by multivariate analysis. Of note, the severity of the GVHD, acute or chronic, was significantly greater in patients with COP compared with controls, and 22 % of the cases were diagnosed in the setting of corticosteroid taper for GVHD [36]. The association with severe acute GVHD and extensive chronic GVHD in survivors beyond day 100 was also shown in a large retrospective study of 193 HCT recipients from a Japanese registry of 9550 allogeneic HCT recipients. Other risk factors that were shown to be associated with COP included donor HLA disparity, female-to-male HCT, related peripheral blood stem cell transplantation and radiation as part of a myeloablative conditioning regimen [38]. Although an alloimmune mechanism may be postulated from the reported association with GVHD, there is currently no direct evidence of a donor T-cell-mediated immune response against lung tissue in this entity.
The clinical presentation of COP after HCT is similar to that in other clinical settings: fever, cough, and progressive dyspnea arising in a subacute to acute tempo. The median time of onset in allogeneic HCT recipients is approximately 3 months after transplant, although many cases are diagnosed after day 100 and in long-term survivors up to several years after transplant. Since the presentation may be subacute and insidious, many patients are diagnosed in the outpatient setting, but the disease can progress to acute respiratory failure necessitating ventilator support. In the aforementioned case series, the median time to diagnosis was 108 days after HCT (range 5–2819 days), after a median symptom duration of 13 days. Pulmonary function testing may reveal a new restrictive defect (43 %), obstructive defect (11 %), mixed restrictive and obstructive defect (8 %), or normal physiology (38 %). Sixty-four percent had a new diffusing capacity deficit [36]. The radiographic hallmark of COP on chest CT is ground glass opacities, with linear opacities in an upper lobe, often bilateral, distribution (Fig. 8.3). Features of biopsy-proven COP on chest CT include ground glass opacities (94 %), consolidation (50 %), and linear opacities (50 %) with an upper lobe predominance (63 %) [39]. These linear opacities are usually not described in COP that occurs in non-HCT settings. Because the radiographic findings as well as the clinical presentation may mimic infectious pneumonia, bronchoscopy with lavage is recommended to exclude infectious etiologies. In cases in which a clinical diagnosis is still unclear, a histologic diagnosis by surgical lung biopsy may be warranted.
The mainstay of treatment for COP after HCT is corticosteroids, usually initiated at a dose of 0.5–1.0 mg/kg/day, depending on the severity. If the patient has severe disease or is in acute respiratory failure, administration of pulse steroids with 1–2 g initial dosing is justified. Recent recommendations suggest treating with corticosteroids starting at 0.75 mg/kg/day until symptomatic or radiographic improvement is noted then tapering slowly over a minimum of 4–6 months [40]. Although there is little more than anecdotal evidence for the use of macrolides in this population, chronic clarithromycin or azithromycin may be added as an adjunctive immunomodulatory agent in patients who have difficulty tapering or tolerating corticosteroids [41].
Overall, prognosis for COP after HCT is good, with a majority of cases resolving or stabilizing with corticosteroids. Relapse is common, particularly when corticosteroid taper is accelerated. A small, but significant proportion of patients will progress in spite of therapy and ultimately succumb to respiratory failure. In refractory cases, it is hypothesized that the interstitial inflammation sets up a process of progressive interstitial fibrosis, evidenced by progressive interstitial changes on computed tomography. It is not known what factors contribute to progression, or which individuals are at risk for progression. As more patients survive past day 100 after allogeneic HCT, there is a sense amongst transplant physicians that COP is increasing in frequency, but the true incidence is difficult to ascertain given the relative rarity of the condition and histologic confirmation by lung biopsy is now rarely undertaken. Investigation into this entity and its impact on HCT outcomes and non-relapse mortality is warranted.
Bronchiolitis Obliterans Syndrome
Bronchiolitis obliterans syndrome (BOS) is a late-onset and chronic pulmonary problem, but it is considered here because acute respiratory failure can occur in the setting of rapid airflow decline and infectious complications. BOS is characterized by new onset irreversible airflow obstruction in the setting of prior or active chronic graft-versus-host disease and usually occurs within the first 2 years after HCT. Although the pathogenesis of BOS is unknown, it is likely a form of lung injury caused by an alloimmune response to the airway epithelium. An aberrant repair response results in the obliteration of small airways with fibrous material, which leads to the physiologic correlate of airways obstruction [42]. The clinical manifestations of BOS are usually insidious, but there is a phenotype of rapidly progressive airflow obstruction which can lead to primary respiratory failure [43]. Even when patients with BOS are stabilized, morbidity and mortality from BOS are high, and result mainly from infectious pneumonia or progressive chronic respiratory failure [42].
Drug-Induced Pneumonitis
Individual components of some conditioning regimens used in HCT are associated with pulmonary toxicity. Carmustine (or bischloroethylnitrosourea, BCNU), used as a single agent or in combination prior to autologous HCT for solid tumor and hematologic malignancies, is associated with acute onset pneumonitis with an incidence of 4–59 %. Prior mediastinal radiation therapy, BCNU dose greater than 1000 mg and age less than 54 were independent risk factors for developing pneumonitis after autologous HCT for lymphoma [44]. The mechanism of BCNU-associated pulmonary toxicity has not been entirely elucidated but is thought to include oxidative stress, glutathione dysfunction and immune-mediated lung injury [45].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree