Fig. 4.1
SEER relative survival rates for AML by age group, 1988–2008. SEER surveillance end epidemiology and end results. http://seer.cancer.gov
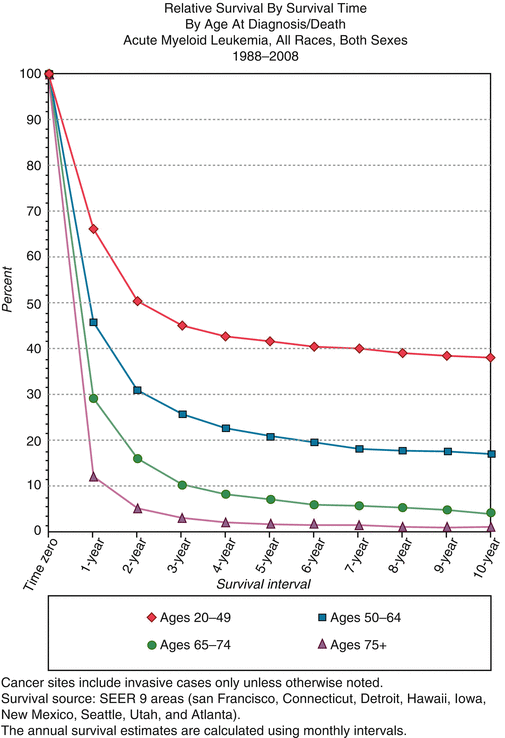
Fig. 4.2
SEER relative survival rates for AML by age group, 1988–2008. SEER surveillance end epidemiology and end results. http://seer.cancer.gov
The primary risk factors for developing AML are older age and a history of prior myelodysplastic syndrome (MDS). Less common risk factors which have been reported include: previous myeloproliferative neoplasm (MPN), chemotherapy drugs (alkylators, topoisomerase 2 inhibitors, and nitrosoureas), radiation or petrochemical exposure.
Current Diagnostic Standards
The clinical signs and symptoms of AML are varied and non-specific but acute in onset. Symptoms are typically related to cytopenias, often pancytopenia due to leukemic infiltration of the bone marrow. As such, patients commonly present with infections, fatigue, or bleeding. Less commonly, older adults with AML may present with severe leukocytosis, producing symptoms of leukostasis which can result in altered mental status (i.e., delirium), shortness of breath, or chest pain. Occasionally presentations related to symptoms of leukemic infiltration of tissues outside the marrow (i.e., liver, spleen, gums, lymph nodes, skin, or central nervous system) are seen.
The diagnosis of AML requires documentation of an abnormal accumulation of leukemic blasts of myeloid origin [3]. The presence of ≥20 % blasts in the marrow or peripheral blood is diagnostic. Exceptions are made for certain genetic abnormalities such as t(8;21), inv(16), t (15;17), t(16;16) and some cases of erythroleukemia. Immunohistochemical and flow cytometry techniques aid morphologic evaluation to confirm myeloid versus lymphoid origin. Conventional cytogenetics is a required component in the diagnostic evaluation of patients suspected of AML with >50 % of patients presenting with a detectable cytogenetic abnormality. Fluorescence in situ hybridization (FISH) for common prognostic chromosomal alterations should be performed if cytogenetic analysis fails. Molecular testing for mutations in NPM1, CEBPA and FLT3 should be considered in cytogenetically normal patients. The role of molecular testing in clinical practice is rapidly evolving. The WHO classification of AML (Table 4.1) highlights the importance of cytogenetic and molecular studies for prognosis and treatment. It has become increasingly clear over recent years that AML comprises a group of distinct diseases with a broad range of tumor biology.
Table 4.1
WHO (2008) classification of acute myeloid leukemias and related precursor neoplasms
Acute myeloid leukemia with recurrent genetic abnormalities |
AML with t(8;21)(q22;q22); RUNX1-RUNX1T1 |
AML with inv(16)(p13.1q22) or t(16;16)(p13.1;q22); CBFB-MYH11 |
APL with t(15;17)(q22;q12); PML-RARA |
AML with t(9;11)(p22;q23); MLLT3-MLL |
AML with t(6;9)(p23;q34); DEK-NUP214 |
AML with inv(3)(q21q26.2) or t(3;3)(q21;q26.2); RPN1-EVI1 |
AML (megakaryoblastic) with t(1;22)(p13;q13); RBM15-MKL1 |
Provisional entity: AML with mutated NPM1 |
Provisional entity: AML with mutated CEBPA |
Acute myeloid leukemia with myelodysplasia–related changes |
Therapy–related myeloid neoplasms |
Acute myeloid leukemia not otherwise specified |
AML with minimal differentiation |
AML without maturation |
AML with maturation |
Acute myelomonocytic leukaemia |
Acute monoblastic/monocytic leukaemia |
Acute erythroid leukaemias |
Pure erythroid leukaemia |
Erythroleukaemia, erythroid/myeloid |
Acute megakaryoblastic leukaemia |
Acute basophilic leukaemia |
Acute panmyelosis with myelofibrosis |
Myeloid sarcoma |
Myeloid proliferation related to Down syndrome |
Transient abnormal myelopoiesis |
Myeloid leukaemia associated with Down syndrome |
Blastoid plasmacytoid dendritic neoplasms |
Tumor Biology
Age-related changes in tumor biology are a major contributor to outcomes for older adults (Table 4.2). Older patients are more likely to have unfavorable cytogenetic abnormalities (i.e., chromosome 5 and 7 abnormalities, and/or complex karyotypes) and less likely to have favorable cytogenetic abnormalities [i.e., t(8;21),t(15;17) and inv(16)] compared to younger patients at the time of presentation [2, 4, 5]. Unfavorable cytogenetic abnormalities are associated with decreased remission rates and shortened overall survival (OS) [2, 4, 6–8]. The expression of a multi-drug resistance phenotype (MDR1) is also more common in older AML patients [9]. This membrane-associated glycoprotein actively pumps out many conventional chemotherapies such as anthracyclines thereby reducing intracellular concentrations and promoting leukemia cell survival. Finally, older adults are more likely to develop AML in the setting previous hematologic disorders (MDS/MPN), which is more resistant to standard therapies [10].
Table 4.2
Selected randomized trials of induction chemotherapy for adults ≥60 years of age with AML
Author | Year | Age (years) | N | Treatment Arms | CR (%) | Median OS (months) | P value for OS | Induction death rate (%) |
|---|---|---|---|---|---|---|---|---|
Standard dose induction | ||||||||
Lowenberg et al. [32] | 1989 | >65 | 31 | Ara-C, daunorubicin, vincristine | 58 | 5.3 | <0.05 | 9.7 |
29 | Supportive care | 0 | 2.8 | N/A | ||||
Lowenberg et al. [37] | 1998 | >60 | 242 | Ara-C, daunomycin | 38 | 9.0 | 0.23 | 6.0 |
247 | Ara-C, mitoxantrone | 47 | 9.7 | 6.0 | ||||
Dose attenuated induction | ||||||||
Tilly et al. [35] | 1990 | >65 | 46 | Rubidazone, Ara-C | 52 | 12.8 | 0.12 | 31 |
41 | Low dose Ara-C | 32 | 8.8 | 10 | ||||
Growth factor support | ||||||||
Lowenberg et al. [41] | 1997 | >60 | 157 | Daunomycin, Ara-C, GM-CSF | 56 | No difference | 0.55 | 14 |
161 | Daunomycin, Ara-C | 55 | 10 | |||||
Stone et al. [39] | 1995 | >60 | 195 | Ara-C, daunorubicin | 54 | 9.4 | 0.10 | 16 |
193 | Ara-C, daunorubicin, GM-CSF | 51 | 9.4 | 20 | ||||
MDR1 modulation | ||||||||
Baer et al. [42] | 2002 | ≥60 | 61 | Ara-C, daunorubicin, etoposide | 46 | No difference | 0.48 | 20 |
59 | Ara-C, daunorubicin, etoposide, PSC-833 | 39 | 44 | |||||
Van der Holt et al. [43] | 2005 | ≥60 | 211 | Daunorubicin, Ara-C | 48 | No difference | 0.52 | Not reported |
208 | Daunorubicin, Ara-C, PSC-833 | 54 | ||||||
Dose Intensification | ||||||||
Lowenberg et al. [44] | 2009 | ≥60 | 411 | Ara-C, daunorubicin 45 mg/m2 | 54 | No difference | 0.16 | 11 |
Addition of gemtuzumab ozogamicin | 402 | Ara-C, daunorubicin 90 mg/m2 | 64 | 12 | ||||
Castainge et al. [45] | 2012 | ≥50 | 139 | Ara-C, daunorubicin, gemtuzumab ozogamicin | 75a | 11 | <0.05 | 4 |
139 | 81a | 28 | 6 | |||||
Lower intensity therapy | ||||||||
Kantarjian et al. [48] | 2012 | ≥65 | 243 | Supportive care or low dose Ara-C | 8 | 5 | 0.2 | 8 |
242 | Decitabine | 18 | 8 | 9 | ||||
Burnett, et al [49] | 2007 | ≥60b | 103 | Low dose Ara-C ± ATRA | 18 | Improved with low dose Ara-C | <0.05 | 26 |
99 | Hydroxyurea ± ATRA | 1 | 26 | |||||
While each of these risk factors independently influences remission rates, the presence of multiple poor risk features dramatically reduces the odds of achieving a complete remission (CR) with standard induction therapies. In a Southwestern Oncology Group (SWOG) study of older adults with newly diagnosed AML treated with intensive induction therapy, patients with none of these adverse risk factors (de novo AML, MDR1 negative phenotype, favorable/intermediate cytogenetics) had a CR rate of 81 % compared to 12 % of those with multiple risk factors (secondary AML, MDR1 positive phenotype, unfavorable cytogenetics) [9]. In another study focused on the small percentage of older patients with favorable cytogenetics [t(8;21), inv(16)], remission rates of 80 % with 30 % 5-year survival were reported [11] resulting in some consensus that this subgroup should be offered intensive therapy if their Eastern Cooperative Oncology Group (ECOG) performance status is <3.
The majority of older adults, however, have cytogenetically normal AML. This group remains molecularly diverse with an ever evolving understanding of the prognostic implications of gene mutations (i.e., FLT3–ITD, NPM1, CEBPA, DNMT3, IDH1 and IDH2, WT1) and overexpression (i.e., ERG, BAALC) [12–18]. The most well defined molecular risk factors are FLT3–ITD mutation (poor prognosis), and NPM1 or isolated CEBPA mutation in absence of FLT3–ITD (good prognosis). The prognostic implications of these mutations are consistent between younger and older AML patients. There are molecular changes that have been observed between older and younger patients. Analysis of gene expression data comparing patients <45 years with those ≥55 years has demonstrated age-specific dysregulation of oncogenic pathways [19]. Current strategies to improve outcomes for older adults include the design of trials using novel agents to target specific molecular AML subtypes. For example, several completed and ongoing trials utilizing FLT3 inhibitors (i.e., midosaurin, sorafenib) in novel combinations were designed to investigate the efficacy of this approach for patients with FLT3 mutations. These trails have so far yielded disappointing results underscoring the genetic complexity of AML in older patients. It is expected that tolerable treatment options will increase for older adults with successful targeting of molecular subtypes of AML and incorporation of genetic signatures into risk stratification schema in clinical trials.
Overview of Treatment Trials for Older Adults with AML
There is ongoing debate regarding the optimal treatment strategy for older adults with AML ranging from supportive care alone to standard aggressive therapies [20–24]. This debate is fueled by low survival rates with substantial risk of treatment toxicity among elders undergoing curative therapies. These concerns translate into low rates of receipt of therapy; less than 40 % of older adults receive chemotherapy after AML diagnosis in the US [25].
Clinical trials have consistently shown worse survival outcomes among older adults with AML compared to younger patients using age cutoffs of 55, 60, and 65 years [2, 26, 27]. Older adults are less likely to achieve a CR and to remain relapse free if they have achieved a CR. For example, in an analysis of SWOG treatment trials, including 968 AML patients, treated with standard induction and consolidation therapy, CR rates were 64, 46, 39, and 33 % in age groups <56, 56–65, 66–75, and older than 75 years, respectively. Among patients with responsive disease the median disease free survival was 21.6, 7.4, 8.3 and 8.9 months, respectively; the OS for the whole population was 18.8, 9.0, 6.9, and 3.5 months, respectively [2]. Older adults are also more likely to experience treatment-related death (typically defined as death within 30 days of initiation of therapy), ranging from 15 to 30 % in clinical trials [2, 21, 22, 28]. These results likely underestimate actual treatment mortality in clinical practice as only patients fit enough to enroll on clinical trial were studied. Due to concerns regarding inferior outcomes with treatment and increased toxicity, a large proportion of older adults in the US are not considered for chemotherapy treatment for this disease [29, 30].
Induction Therapy
Standard induction chemotherapy for non-acute promyelocytic (APL) AML typically involves a treatment regimen that includes cytarabine (Ara-C) and an anthracycline given for 7 and 3 days respectively (7 + 3) [31]. Lowenberg et al. performed a landmark study demonstrating survival improvement for patients ≥65 years of age treated with intensive induction therapy versus supportive care alone [32] with no difference in time spent hospitalized between the two groups. This study provided supporting evidence to offer curative chemotherapy to selected older adults with AML. Population-based data from Sweden and the US similarly show a survival advantage for treatment [25, 33].
In the decades since publication of the landmark Lowenberg trial, many subsequent elderly- specific studies have been conducted. Unfortunately, most have failed to improve upon the suboptimal treatment outcomes seen in the older population (Table 4.2). In most elderly-specific trials, CR rates range from 40 to 60 %, but median survival remains less than a year [22]. Several variations of induction therapy regimens designed to improve the balance of efficacy versus toxicity have been investigated. Dose-attenuation, to minimize toxicity, has not resulted in substantial improvement in outcomes [34, 35]. Investigation of various anthracyclines, such as mitoxantrone and idarubicin, and the addition of etoposide have not improved survival for older adults [28, 36–38]. Use of growth factors including granulocyte colony stimulating factor (G-CSF) and granulocyte macrophage colony stimulating factor (GM-CSF) to decrease duration of neutropenia has not consistently been shown to improve response rates or survival; likewise they do not clearly decrease costs [28, 36, 39–41]. Modulation of the multi-drug resistant efflux pump (MDR1) was pursued using a cyclosporine analog PSC-833 with standard induction chemotherapy in two randomized trials [42, 43]. There was no benefit in response rates or survival and an increased toxic death rate on the experimental arm of the Cancer and Leukemia Group B trial [42].
Investigation of high anthracycline dose intensity has led to a positive outcome in a subset of older adults. Specifically, 90 mg/m2 of daunorubicin improved CR rates compared to 45 mg/m2 (64 % versus 54 % respectively) for untreated older adults receiving a 7 + 3 regimen [44]. While there was no significant increase in toxicity with higher dosing, the improved CR rate did not translate into better OS. In subset analyses, benefits appeared limited to adults aged 60–65 years. In this subset of “young-old” patients, a survival benefit was noted. No randomized trial data is available regarding comparison of the commonly used 60 mg/m2 dosing with 90 mg/m2. Two studies have shown positive outcomes when adding gemtuzumab ozogamicin to induction therapy for AML. Gemtuzumab ozogamicin is a humanized anti-CD33 monoclonal antibody linked to calicheamicin which has demonstrated efficacy in the relapsed setting but its use in older adults was after it was voluntarily withdrawn from the US market following a negative trial in younger AML patients. Recent investigations, however, testing a lower dose schedule in combination with intensive induction, suggest that the combination may be efficacious and tolerable. A phase 3 study of 280 adults aged 50–70 with untreated AML showed improvement in relapse-free (23 % versus 50 %) and overall survival (42 % versus 53 %) with the addition of gemtuzumab [45]. Benefit was most evident among patients with favorable cytogenetics. A larger study of patients >50 years old with AML or high risk MDS similarly showed a survival advantage to the addition of gemtuzumab to two different induction regimens without significant increased toxicity [46]. These studies have renewed interest in use of this agent in the curative setting, particularly among those with good risk cytogenetics, although data remains limited for adults >70 years of age. At present, gemtuzumab remains unavailable in many healthcare systems.
Lower intensity regimens have been tested for older adults, particularly for those considered “unfit” for intensive chemotherapy or presenting with more indolent disease. Use of DNA hypomethylating agents (azacitidine and decitabine) has increased significantly in recent years [25]. These agents have demonstrated activity in MDS and subset analyses of patients with low blast count AML (20–30 %) included on MDS treatment trials show evidence of increased response and survival compared to standard care (a mix of low dose Ara-C, best supportive care or 7 + 3) [47]. A multi-center trial of decitabine versus supportive care or low dose Ara-C for adults ≥65 years with intermediate or poor risk cytogenetics reported a CR rate of 18 % for decitabine and, in an unplanned post-hoc analysis, a small survival benefit (median 7.7 versus 6.2 months) [48]. At present, neither drug is approved for treatment of AML by the US Food and Drug Administration but both have an indication approved by the European Medicines Agency. Another low intensity option is low dose Ara-C which has limited activity and can improve survival among patients not fit for intensive chemotherapy compared to supportive care [49]. Low intensity regimens have not been compared in a randomized fashion to intensive therapy for older adults nor have rigorous definitions of fit or unfit been used in clinical trials to optimize extrapolation of data in clinical practice.
Post-remission Therapy
Optimal post-remission strategies for older adults are also undefined [31, 50]. It is generally accepted that post-remission therapy or consolidation is needed to translate a complete remission into cure [31]. Typical post-remission treatment includes high dose cytarabine or hematopoietic stem cell transplantation. For older adults with favorable or intermediate risk AML in first CR, consolidation chemotherapy without transplant is typically pursued. In a randomized study of low, intermediate and high dose cytarabine for consolidation in patients who had achieved CR, younger patients benefited from dose escalation while older patients experienced more cerebellar toxicity and no benefit in disease free survival [27]. For younger patients, high dose cytarabine (3 g/m2) is a standard post-remission treatment option for good risk cytogenetics [31]. This treatment is too toxic for older adults and likely contributes to suboptimal outcomes seen in older adults with good risk cytogenetics. In elderly specific trials, lower dose Ara-C regimens as a single agent or in combination with an anthracycline have been tested [36, 51]. In this population, there is no clear evidence to date that multiple courses of consolidation or maintenance therapy improve outcomes when compared to a single course of consolidation therapy [34, 36, 51].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree