Fig. 6.1
Incidence of acute myelogenous leukemia (AML) by sex and single years of age in children and AYAs (Surveillance, Epidemiology, and End Results, US SEER 18 regions, 2000–2011)
Incidence trends: According to SEER 2000–2012 [53], the incidence of AML has been stable since the late 1970s in AYAs, whereas in younger and older persons, the incidence has increased since the late 1970s. The German Childhood Cancer Registry [35] and the Nordic countries [42] have not reported an increase in the incidence in AML in children under 15 years. In Great Britain there is a small increase in incidence since 1975 (all leukemia subtypes combined) in most age groups (mainly in the elderly over 65), but not in the 15–24 age group. These trends have to be interpreted with caution, because changes in the diagnosis, classification, and registration are likely to explain at least some of the observed increase. Over the last decade, incidence rates remained relatively stable [60].
6.2.2 Etiology
There are only a few proven etiologic factors for childhood AML, for example, in utero exposure to alcohol, exposure to benzene, ionizing radiation, or different drugs that may contribute to AML in young children. The risk of AML is increased in children with congenital syndromes such as Fanconi anemia, Shwachman-Diamond syndrome, and Down syndrome. Somatic mutations of the GATA 1 gene are seen in virtually all cases of AML associated with Down syndrome and may be implicated in the 500-fold increased risk of megakaryoblastic AML seen in these patients [22, 31]. Such mutations may also confer enhanced leukemic sensitivity to cytarabine via dysregulation of cytidine deaminase gene expression [27]. AML as a secondary malignancy after intensive chemotherapy is quite often seen in older children and adults (cumulative incidence of 0.6 % for children treated for ALL or solid tumors by 10-year follow-up and 3.3–10 % for adults treated for different types of solid tumors) [38, 43].
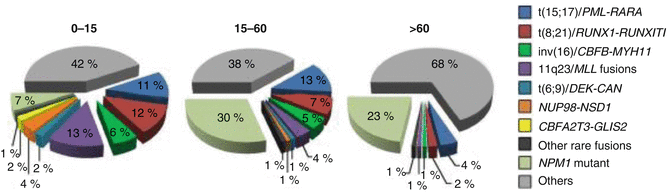
AML is the most common and more likely to occur than ALL in the older age groups (especially >60 years old), correlating to prolonged duration of exposure to environmental carcinogens proportional to age and accumulation of mutations from genetic error events in cell division [8]. Only the incidence of acute promyelocytic leukemia (APL, t(15;17)/PML–RARA, WHO-2008 classification) appears approximately constant with respect to age after the first decade [62]. According to SEER data, APL had its highest proportion among subtypes of AML in AYAs, with 20–25 % of those with AML having the APL subtype (Fig. 6.2). A report from Japan ascertained a gradual increase of APL as a proportion in adolescence: 1–4 years, 5 %; 5–9 years, 8 %; 10–14 years, 12 %; 15–19 years, 19 %; 20–24 years, 22 %; 25–29 years, 21 % [33]. A more recent report from this group analyzing diagnoses from 2006 to 2010 again confirmed that APL made up an increasing proportion of AML diagnosis from childhood through adolescence (infants, 1.8 %; 1–4 years, 3.9 %; 5–9 years, 10 %; 10–14 years, 12 %; 15–19 years, 15 %) [32]. Acute promyelocytic leukemia shows a high frequency (20–24 % of AML diagnoses) in certain ethnic populations (e.g., Italian and Latin American) compared to other ethnic groups (5–8 %). In a single institute in Mexico, 20 % of all AML patients and 30 % of adolescents (11–21 years old) presented with APL [49]. This may suggest a genetic predisposition for acute promyelocytic leukemia and/or specific environmental exposures [25].
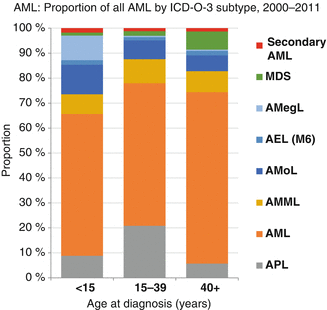

Fig. 6.2
Proportion of all AML by ICD-O-3 subtype, SEER 2000–2011
6.2.3 Trends in Survival
The 5-year AML-specific survival rate has doubled in all age groups since the late 1970s. Also, the rate of progress in improving the 5-year AML-specific survival rate is similar in children, AYAs, and older adults [53] (Fig. 6.3).
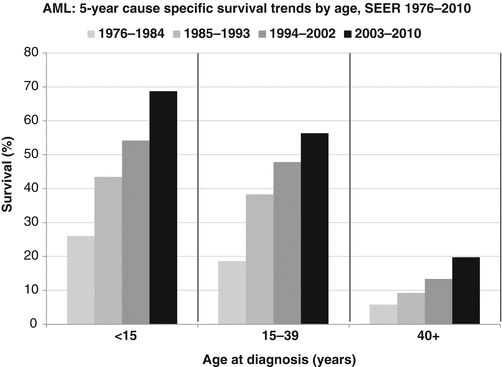

Fig. 6.3
5-year cause-specific survival trends by age, SEER 1976–2010 (Source: SEER 9 Areas, NCI)
Survival rates for AYAs with AML have improved over the last three decades. Population-based estimates of 5-year survival increased from 18.6 % in the period 1975–1984 to 56.3 % in the period 2003–2010 [53]. Current 5-year survival in children, adolescents enrolled in clinical trials (which tend to be higher estimates because trials may exclude patients with unfavorable features or patients from small hospitals), is in the range of 60–75 % [18]. Results from the AML-Berlin-Frankfurt-Münster (BFM) studies (patients <18 years old) showed an improvement of 5-year survival from 49 % (study AML-BFM 87, in the period 1987–1992) to 60 % (period, 1993–1998), to 65 % (period, 1999–2003) and to 75 % (period, 2004–2010) [19, 21]. In young adults, 5-year survival rates are now in the range of 50 % according to population-based data [34]. In clinical trials the 10-year survival rate in the recent German-Austrian AML Study Group (AMLSG) studies is reported as 50–60 % for 18–45 years old patients [24].
The improvement in prognosis over the last decades in all age groups became possible by intensified chemotherapy in de novo AML and after relapse and supportive care. With intensive induction chemotherapy, 80–90 % of young patients achieve complete remission (CR).
Little data specifically analyzes overall survival (OS) for adolescents and young adults. Population-based data from regions of England and Wales showed that a 5-year survival improved significantly from 36 % in the period 1984–1988 to 46 % in the period 1989–1994 and to 56 % in the period 2001–2005 for AML patients between 15 and 29 years old (although the 2001–2005 period included only patients aged 15–24 years) [54, 61].
According to SEER data, 2000–2010 AYAs had nearly as good as a 5-year survival as younger patients (Fig. 6.4) [53]. Above age 40, the survival rate declined more rapidly.
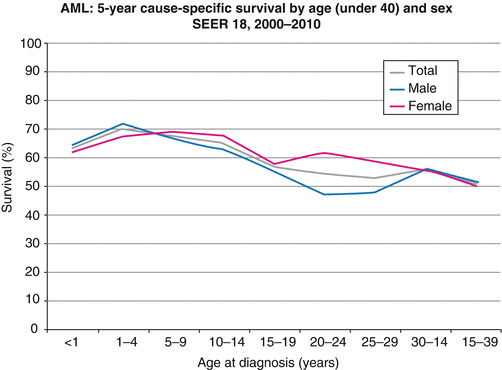

Fig. 6.4
5-year AML-specific survival by age (under 40) and sex, SEER 2000–2010 (Source: National Cancer Institute: SEER18 Areas)
6.2.4 Prognostic Factors
The 5-year AML-specific survival was similar in males and females, except for a lower rate in 20- to 29-year-old males (Fig. 6.4).
Whereas non-Hispanic white AYAs had an inferior survival in comparison to younger patients, other common races/ethnicities had relatively similar survivals in AYAs and younger patients [53]. However, this may be related to the lower incidence of APL.
Increasing age is a known poor prognostic factor in adults with AML [11, 17, 34]. In population-based studies, 5-year survival rates drop with age, but are now 64 % for patients aged 0–15 years and about 54 % for those aged 15–40 years (Fig. 6.4) [53]. However, prognosis in different age groups of children and older adolescents treated similarly has rarely been reported and is often conflicting.
Results of the former Children’s Cancer Group (CCG) trials (CCG 213 [1986–1989] and CCG-2891 [1996–2002]) including children and adolescents less than 22 years old were conflicting with no difference in survival (5-year survival was 39 % and 50 %, respectively) in 2–10-year-old children and adolescents aged 10–21 years [63, 64]. However, in patients above 16 years event-free survival (EFS) and survival were inferior in the last CCG-2891 trial [36].
Recently, a cross-study analysis combining data from the CCG-2891, CCG-2941, CCG-2961, and AAML03P1 Children’s Oncology Group (COG) trials showed that survival in AYA and younger patients with newly diagnosed AML was similar. However, older patients were at higher risk for treatment-related mortality (TRM): Overall survival in AYAs (16–20 years, n = 238) was 49 ± 7 % versus the younger (n = 1602, 54 ± 3 %, P = 0.058); relapse was lower in AYA patients (30 ± 7 % versus 41 ± 3 %, P = 0.002), but TRM was higher (25 ± 6 % versus 12 ± 2 %, P < 0.001). Infection accounted for the excess TRM in AYA patients [16]. The COG also demonstrated similar outcomes for AYAs when combining data from the most recent trials AAML03P1 and AAML0531, which tested adding gemtuzumab ozogamicin to a MRC-based chemotherapy regimen. AYA patients aged 16–21 years compared to those <16 years had similar 5-year EFS (44.2 % vs. 50.2 %) and 5-year OS (60 % vs. 64.8 %). AYA patients were again found to have higher rates of TRM 13.3 % (compared to 7.3 % in younger patients, P = 0.005). Further, AYA patients who received gemtuzumab ozogamicin or stem-cell transplant had higher rates of TRM that appeared to attenuate the survival advantage seen with these interventions among young patients [2].
Similar results were reported by Rubnitz et al. when analyzing outcomes from the St. Jude AML protocols AML91, AML97, and AML02 covering the period of 1991–2008. The survival rate for older children with AML has improved in the recent trial (AML02) and was now similar to that of younger patients. However, deaths from toxicity remained a significant problem for patients in the older age group [48].
Unfortunately, no recent data for the AYA group are available from the British Medical Research Council (MRC) trials. In a study of MRC AML12 in <16-year-old children, 10-year EFS and OS were 54 % and 63 %, respectively [28]; in <60-year-old adults, OS at 8 years was 38 % [13].
The same trend to decrease in survival with age was reported for the EFS rates but not overall survival in more than 1,000 Japanese AML patients aged 1–29 years consecutively diagnosed in the period 1986–1999, who were treated in a variety of institutions and protocols. Seven-year probability of EFS for AML decreased from 34 % in the age groups 10–15 years to 32 % for 15- to 19-year-old adolescents and to 26 % in the 20- to 29-year-old young adults [33]. Recently, lower survival but not EFS rates were reported in Japanese patients aged 15–17 years compared to younger patients [58]. This was mainly due to a higher treatment-related death rate after relapse in AYAs.
Treatment schedules and dosing of the AML-BFM 93/98 studies for children and adolescents (n = 891) and the AMLCG92/AMLCG99 and AMLSG HD93/AMLSG HD98A studies for adults were similar during induction and consolidation [10, 12, 51, 52]. In the adult studies, 290 patients were 16–30 years old. A common analysis of patients of the studies showed that the CR rate was highest in the age groups 2–12 years (89 %) and lower in infants and patients of older age (<2 years, 79 %; 13 ≤ 21 years, 82 %; 21–30 years, 72 %). Long-term treatment results were also most favorable among 2–12-year-old children (5-year EFS ± SE, 53 ± 2 %), slightly inferior in adolescents (45 ± 3 %, P = 0.03), and unfavorable in young adults (28 ± 3 %, P = 0.0001) (for more details, see Table 6.1). Excluding patients with low-risk cytogenetics [t(8;21), inv16, and t(15;17)], results were inferior in adolescents (EFS 39 ± 5 %) and young adults (EFS 25 ± 6 %) compared with children aged 2–12 years (EFS 52 ± 4 %) [16].
Table 6.1
Results in patients treated in pediatric and adult trials according to age groups
Age (years) | <2 n (%) | 2 ≤ 13 n (%) | 13 ≤ 21 n (%) | 21 ≤ 30 n (%) | p (gray) |
|---|---|---|---|---|---|
Total of patients | 222 | 463 | 276 | 220 | |
Early death (ED)a | 16 (7.2) | 17 (3.7) | 13 (4.7) | 14 (6.4) | |
Nonresponse (NR) | 31 (14.0) | 36 (7.8) | 36 (13.0) | 47 (21.4) | <0.001 |
Death in CCR (cumulative incidence) % (SE) | 2 (1) | 4 (1) | 5 (2) | 6 (3) | <0.093 |
Complete remission | 175 (78.8) | 410 (88.6) | 227 (82.2) | 159 (72.3) | |
Relapse (cumulative incidence) % (SE) | 35 (5) | 30 (3) | 32 (4) | 38 (6) | 0.098 |
5-year EFS % (SE) | 42 (3) | 53 (2) | 45 (3) | 28 (3) | <0.0001b |
5-year survival % (SE) | 57 (3) | 62 (2) | 56 (3) | 42 (4) | <0.0001b |
6.2.5 Treatment Differences
Adolescents and young adults may be treated on pediatric or adult trials. Recently, 281 adolescents 16–21 years old treated on the pediatric cooperative trials groups CCG and COG trials (1986–2008) were compared with 149 patients of the same age group treated on the adult cooperative trials groups Cancer and Leukemia Group B (CALGB) and Southwest Oncology Group (SWOG) frontline AML trials (1986–2008). Patient characteristics were similar; however; age was a confounding variable (median, COG: 17.2 versus CALGB: 20.1 and SWOG: 19.8 years, P < 0.001). The 10-year survival rate for patients treated on the CCG/COG trials was 45 ± 6 % compared to 34 ± 7 % in the adult trials, P = 0.026 [65].
6.3 Biology/Pathology
Biological parameters across the entire age spectrum are reported rarely in literature. The frequency of cytogenetic subgroups of AML is age specific. There is an increase of the poor prognostic cytogenetic groups (e.g., unbalanced aberrations) in adults with older age (Fig. 6.5) [3, 29].
Clinical, morphological, and cytogenetic data were analyzed for children, adolescents, and young adults treated in the pediatric trials AML-BFM 93/98 (n = 869) and of 92 young adults (<30 years) of the AMLCG92 study. Age classifications were infants (≤2 years), children between 2 and 12 years of age (because there were significant differences in biological parameters in these age groups), adolescents between 13 and 21 years of age, and young adults between 21 and 30 years [20]. Results show that French-American-British (FAB) distribution was quite different in young children <2 years, 68 % (147/213), who presented with FAB subtypes M5 or M7, compared to 18 % (133/730) in the older age groups (χ 2 P < 0.0001) (Fig. 6.6). However, apart from a trend toward increasing M1 and decreasing M7, there was no difference in FAB types for children (2–12 years) and AYA patients 13–30 years old. The favorable karyotypes t(8;21), t(15;17), and inv16 were rarely seen in children <2 years (8/163 = 5 %) compared to the 2- to ≤21-year-olds (141/580 = 24 %; Fisher P = 0.01; Table 6.2). With the limitation of the low patient number, it is of interest that t(8;21) was seen less frequently in young adults aged 21–30 years compared to the 2–21-year-old group.
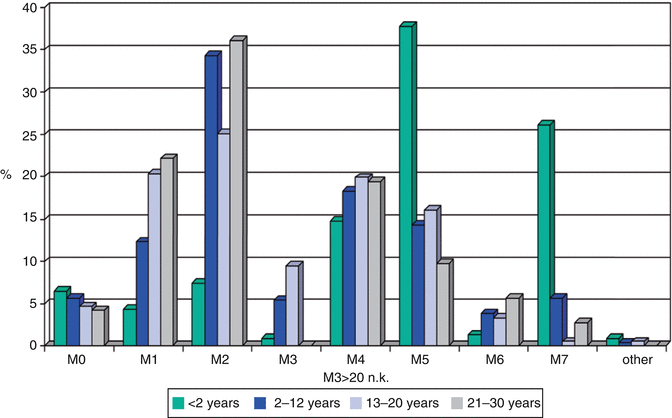
Fig. 6.6
Distribution of FAB (French-American-British) classification subtypes of AML as a function of age group (Data from the AML-BFM studies 93/98 and AMLCG92)
Table 6.2
Karyotypes in the different age groups
Age (years) | <2 | 2–12 | 13–21 | 21–30 | p (χ 2) |
|---|---|---|---|---|---|
t(8;21) (%) | 1 | 18 | 10 | 5 | 0.0001 |
t(15;17) (%) | 2 | 6 | 8 | 10 | 0.02 |
inv16 (%) | 2 | 8 | 6 | 9 | 0.07 |
11q23 (%) | 27 | 11 | 7 | n g | 0.0001 |
Total (n) | 164 | 320 | 150 | 43 |
These data do not show significant differences in biological parameters between children 2–12 years old compared to AYAs, albeit there was a lower incidence of 11q23 and t(8;21) above age 12 years than below this age. Only patients younger than 2 years of age present with significant differences in comparison to older patients. APL, which is characterized by t(15;17), does vary as a proportion of AML diagnoses across age groups as discussed above in Sect. 7.3.2.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree