Fig. 7.1
ALL incidence by single years of age, 2000–2012, SEER 18 areas and area-under-the-curve (yellow) that represent “AYA ALL.” The inset shows the same data on an enlarged y-axis for 15- to 39-year-olds. The number of AYA patients is annual average for 2000–2012 in the USA. “Other ALL” refers to types of ALL that occur predominantly in children or older adults
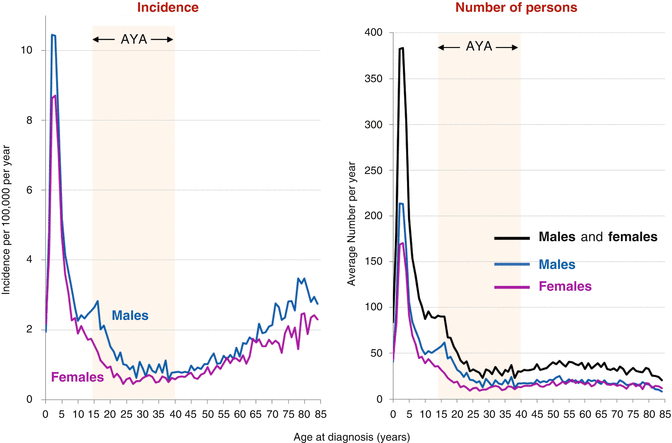
Fig. 7.2
ALL incidence and estimated number of Americans by single years of age and sex, 2000–2011, SEER 18 areas
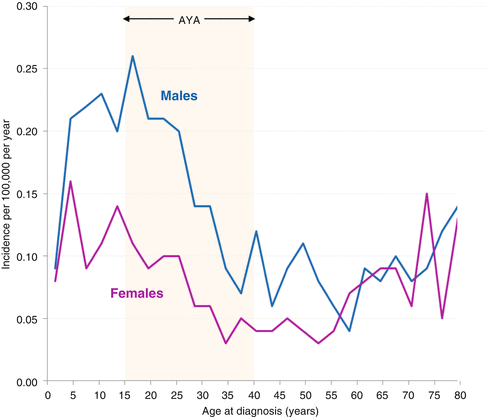
Fig. 7.3
Lymphoblastic lymphoma: incidence by 3-year age intervals and sex, SEER 18 areas
Since 1975 in the USA, there has been an increasing incidence of ALL in all age groups (Fig. 7.4), especially in the AYA age group and particularly in females, which has continued to date (Fig. 7.5). In 20- to 49-year-olds, the incidence increased at a steady rate of 2.9 % per year in females and 1.5 % per year in males (Fig. 7.5, inset). The estimate of an average 1,000 new diagnoses of ALL per year in AYAs in the USA during 2000–2012 is likely considerably greater today, since the incidence in the age group has been steadily increasing.
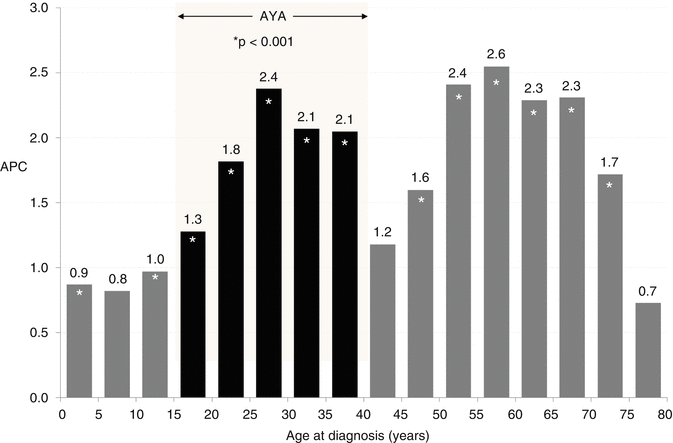
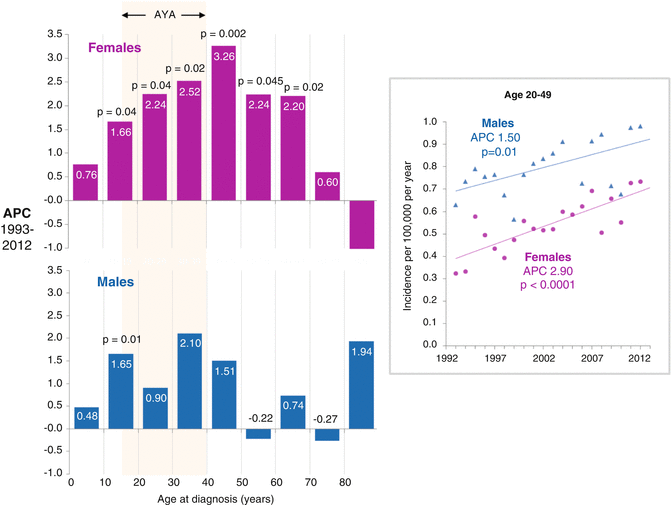

Fig. 7.4
ALL: annual percent change (APC) in incidence and age, 1975–2011, SEER 9 areas

Fig. 7.5
ALL: annual percent change (APC) in incidence by sex and age, 1993–2012, SEER 13 areas. Inset annual incidence of ALL in 20- to 49-year-olds, by sex
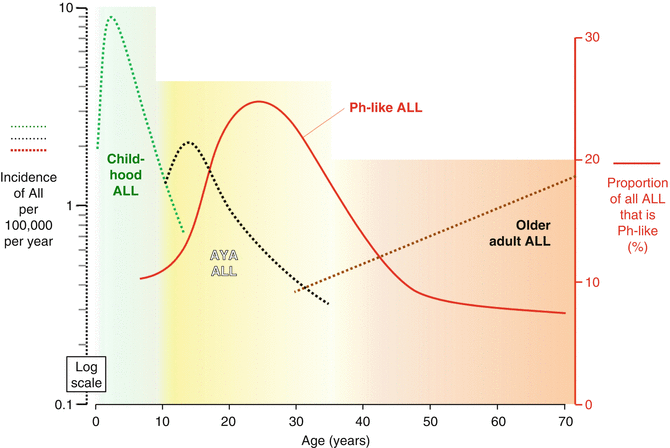
In 2009, two groups identified a subset of B lymphoblastic leukemia (B-ALL) with a gene expression profile similar to that of Ph+ B-ALL, but without the presence of the BCR-ABL fusion protein [1, 2]. This entity has become known as Philadelphia-like (Ph-like) or BCR–ABL1-like ALL and occurs most often in the AYA population (Fig. 7.6). Roberts et al. reported an incidence of 21 % in adolescents (16–20-year-olds) and 27 % in young adults (21–39-year-olds), but only 10 % of children between the ages of 1 and 9 years harbored Ph-like disease [3]. This peak in the AYA population was confirmed in a German cohort of adolescents, young adults, and older adults, in which the incidence of Ph-like ALL was also highest in the AYA age group, and decreased with advancing age [4]. Philadelphia-like ALL is more common in males than in females [3], peaking in 20–30-year-olds. This may account for some of the incidence and outcome discrepancies between AYA males compared to females.
7.1.2 Survival and Mortality
Since 1975, the survival rates for pediatric, adolescent, and young adult patients with ALL have increased, but as of 2006, still fewer than half of AYAs survived 5 years from diagnosis (Fig. 7.7) [5]. Based on SEER data, from 2000 to 2011 the 5-year relative survival rate of ALL declined most strikingly between the ages of 16 and 21 years (Fig. 7.8); this was true both for males and females. Additionally, the average annual percent change (APC) in death rate declined from 1998 to 2011 in patients of all ages diagnosed with ALL, except for young adults aged 25–45 years (Fig. 7.9).
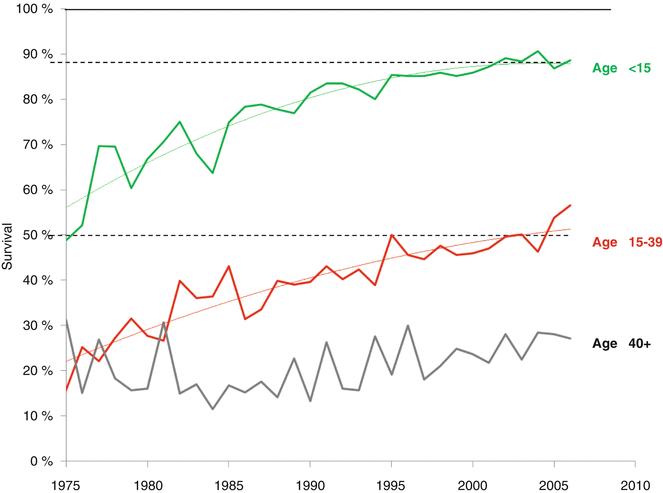
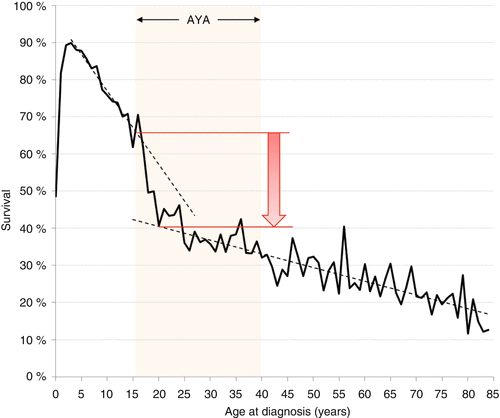
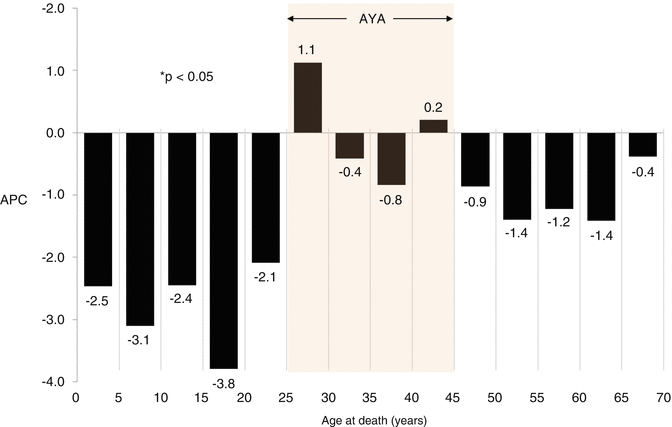

Fig. 7.7
Annual 5-year relative survival by age, 1975–2006, SEER 9, 13, and 18 areas. The regressions are 1° polynomials

Fig. 7.8
ALL: 5-year relative survival, 2000–2011, at 1-year age intervals, SEER 18 areas. Arrow indicates 25 % absolute drop in 5-year survival from age 16 to 21. Dotted lines indicate linear regressions for ages 3–17 and 25–84 years

Fig. 7.9
ALL: annual percent change (APC) in death rate by age, 1998–2011. The data are from the National Center for Health Statistics
These trends are concerning, but they may be partially attributable to population-based reporting, since several groups have reported better outcomes for patients treated on clinical trials. From 1996 to 2001, the Children’s Cancer Study Group (CCG) enrolled patients 1–21 years old with NCI high-risk disease (white blood cell [WBC] >50 × 103/μL or age ≥10 years at diagnosis) onto study CCG-1961 [6]. The 5-year event-free survival (EFS) for patients 16–21 years old was 71.5 % (SE, 3.6 %) and the 5-year overall survival (OS) was 77.5 % (SE, 3.3 %). St. Jude Children’s Research Hospital (SJCRH) conducted the Total Therapy Study XV from 2000 to 2007 [7]. Of the 498 patients that were enrolled, 45 were older adolescents (15–18 years old) who had a 5-year EFS of 86.4 % (95 % CI, 72.1–93.6 %) and a 5-year OS of 87.9 % (95 % CI, 73.1–94.9 %). During a similar time frame, the Medical Research Council (MRC) enrolled patients 1–24 years of age on the UK ALL 2003 trial [8, 9]. While the outcomes for the AYA patients were not reported separately from those of the younger children, for all evaluable patients, the 5-year EFS was 87.3 % (95 % CI, 86.1–88.5 %), and 5-year OS was 91.6 % (95 % CI, 90.6–92.6 %).
The AYA patients enrolled on these clinical trials appear to have had better outcomes compared to the population of AYAs with ALL as a whole, as reported to SEER. This provides support for referral of AYAs with cancer to centers participating in AYA-inclusive therapeutic clinical trials, where there is experience treating this distinctive group of patients. In a population-based cohort of 4,336 children, adolescents, and young adults who were treated for a variety of hematologic malignancies between 1998 and 2008, AYAs between the ages of 15–39 years with ALL who were treated at community sites had worse outcomes than children between the ages of 10–14 years [10]. That difference was abrogated for 15–21-year-olds and greatly diminished for 22–39-year-olds though, if AYAs were treated at NCI Comprehensive Cancer Centers or Children’s Oncology Group (NCICCC/COG) Institutions. Of note, in this study population, 69 % of children were treated at NCICCC/COG sites, but only 38 % of 15–21-year-old patients and 10 % of 22–39-year-old patients were treated at such centers.
Even on clinical trials though, AYAs with ALL are not faring as well as their younger counterparts [11, 12]. The discrepancy in survival between children and AYAs is certainly multifactorial, but disease biology is unquestionably important. As noted, up to 30 % of AYAs with ALL can be identified as having Ph-like ALL, and these patients have a particularly poor prognosis compared to patients without this gene expression signature [1, 2, 13]. In the study by Roberts et al. [3], which included more than 2,000 patients, adolescents with Ph-like ALL had a 5-year EFS and OS of 41.0 ± 7.4 % and 65.8 ± 7.1 %, respectively, compared to 83.3 ± 3.6 % and 92.5 ± 2.5 % in similarly aged patients with non-Ph-like ALL (p < 0.001 for both comparisons). For young adults with Ph-like ALL, the 5-year EFS and OS were 24.1 ± 10.5 % and 25.8 ± 9.9 %, outcomes which were significantly worse than young adults with non-Ph-like ALL, in whom the 5-year EFS and OS were 63.1 ± 9 % and 75.4 ± 8.2 %, respectively (p < 0.001).
Ultimately, survival outcomes for AYAs with ALL are improving, but continue to lag behind that of younger children. Contributing factors likely include, but are not limited to, disease biology, treatment location, and therapeutic regimens. A growing appreciation for the specific therapeutic and psychosocial needs of this age group is expected to lead to further improvements in survival rates.
7.2 Clinical Presentation and Genetics
As a deeper understanding of the biology of ALL is developed, it is evident that AYAs present with higher-risk biology compared to their younger counterparts. The T lineage immunophenotype (T-ALL) is more common in the AYA population [14] and previously portended a worse prognosis, although with current therapies outcomes are approaching those of patients with B-ALL [15–18]. In fact in some adult ALL studies, patients with a T-cell immunophenotype fared better than patients with B-cell disease [19, 20]. Cytogenetic abnormalities, an important component of all risk stratification systems, shift toward more unfavorable profiles with increasing patient age [21]. The leukemic blasts in up to half of children between the ages of 1 and 10 years with B-ALL harbor either the favorable t(12;21) translocation or trisomies of chromosomes 4, 10, and 17, but these findings are rare in patients 20 years of age and older [11, 22]. Conversely, the incidence of intrachromosomal amplification of chromosome 21 (iAMP21) is more commonly found in the AYA and older adult population compared to younger patients [22–24], as is the t(9;22) translocation, which results in the Philadelphia chromosome, or BCR-ABL fusion protein [22]. It is important to note, however, that even though patients with Ph+ ALL are considered to have particularly aggressive disease, outcomes have been substantially improved by the incorporation of tyrosine kinase inhibitor therapy onto cytotoxic chemotherapy backbones [25–27].
As described earlier, Ph-like ALL peaks in the AYA age group [1–4]. The Ph-like subset of B-ALL is characterized by deletions of IKZF1 as well as aberrations in cytokine receptor and tyrosine kinase pathways [28]. Overexpression of cytokine receptor-like factor 2 (CRLF2) is particularly common in cases with IKZF1 deletion and is correlated with JAK mutations and constitutive activation of the JAK-STAT pathway [29–31]. Using next-generation sequencing techniques, several other rearrangements (ABL1, JAK2, PDGFRB, CRLF2, and EPOR), mutations (IL7R and FLT3), and deletions (SH2B3, a gene which encodes a negative regulator of JAK2) have been identified in Ph-like ALL [32], many of which (ABL1, ABL2, CSF1R, PDGFRB, CRLF2, JAK2, EPOR, IL2RB, NTRK3, PTK2B, TSLP, and TYK2) are predicted to respond to various tyrosine kinase, JAK, or other signaling pathway inhibitors [3]. The relatively higher incidence of Ph-like ALL in AYA patients compared to younger and older counterparts may at least partially explain the lack of improvement in outcomes in these patients, and the effects of the incorporation of targeted therapies on such outcomes remains to be seen.
7.3 Treatment
7.3.1 The “Pediatric” Approach
The pediatric approach to ALL treatment relies on high cumulative doses of nonmyelosuppressive drugs, such as vincristine, glucocorticoids, and asparaginase, to be given during periods of neutropenia induced by anthracyclines, alkylators, and antimetabolite chemotherapy [15, 17, 18, 33, 34]. Most cooperative groups use an approach pioneered by the Berlin-Frankfurt-Münster (BFM) study group, with each group having made modifications based on the nuances of their own approach to risk stratification and their approach to treating particular subsets of patients. Remission induction (referred to as “protocol IA” in Europe) includes vincristine, prednisone or dexamethasone, asparaginase, and daunorubicin and is followed by consolidation therapy/protocol IB consisting of cyclophosphamide, cytarabine, and 6-mercaptopurine (6MP). Following these initial intense months, there is a phase termed “interim maintenance” (IM) in the USA, or “protocol M” in Europe, which consists largely of intravenous methotrexate (MTX) given in intermediate or high doses with leucovorin rescue, in conjunction with oral 6MP, and in the USA intravenous vincristine is administered as well. Subsequently, there is a return to intense therapy (“delayed intensification” in the United States, “protocol II” or “protocol III” in Europe) which is essentially re-induction and reconsolidation phases. Typically at this time, prednisone is replaced by dexamethasone, daunorubicin by doxorubicin, and 6-mercaptopurine by 6-thioguanine. On some US protocols, a second IM phase may be administered, but this time using vincristine, escalating doses of intravenous MTX without rescue, and asparaginase (“Capizzi methotrexate”). Debate continues regarding the benefits of this second IM phase. All protocols call for a prolonged maintenance phase that relies mainly on antimetabolite therapy. Dedicated treatment of the central nervous system (CNS) with intensive intrathecal chemotherapy begins during induction and continues throughout therapy. On many protocols, patients with overt CNS disease at diagnosis or with certain very high-risk features are also treated with cranial irradiation.
While there is a discrepancy between improvements in outcomes between younger children compared to the AYA age group, adolescents on cooperative group studies fare better now than in previous eras. On Children’s Oncology Group (COG) ALL studies from 1990 to 2005, the 5-year OS for patients 15–22 years of age improved from 66.1 ± 2.3 % in the early 1990s to 75.9 ± 2.6 % in the early 2000s. Also improved was the percentage of adolescents diagnosed with ALL who enrolled onto clinical trials. From 1990 to 2005, 33.5 % of adolescents aged 15–19.99 years predicted to develop ALL were enrolled onto COG trials. Heightened awareness of the issues surrounding AYA oncology and the importance of enrollment on clinical trials led to that number increasing to 51 % by 2009 [12]. Similarly, good results have been reported by other groups. For adolescents treated on Dana-Farber Cancer Institute (DFCI) protocols 91-01 and 95-01, 5-year EFS was 77 % (SE, 4 %) and 78 % (SE, 6 %), and 5-year OS was 78 % (SE, 4 %) and 81 % (SE, 6 %) for 10–15- and 15–18-year-olds, respectively [35]. DFCI protocols include frequent doses of vincristine, asparaginase, and corticosteroids, as well as high cumulative doses of anthracyclines. Untoward toxicities were not observed in the adolescent patients, although there were higher rates of pancreatitis and thrombosis compared to patients <10 years old [35].
The importance of enrollment onto clinical trials cannot be stressed enough. Treatment regimens for ALL are prolonged and complex, and adherence to therapy is crucial. Providers must be familiar with the entirety of the treatment, and must be vigilant in administration of chemotherapy according to the prescribed schedule. Medical professionals who care for AYAs with ALL must also recognize the challenges faced by AYAs as they attend frequent clinic visits while maintaining educational and employment responsibilities, and who struggle to comply with oral chemotherapy schedules while simultaneously exerting independence and autonomy from parents or other guardians. Uniform treatment of large numbers of patients on clinical trials will continue to raise awareness of appropriate treatment regimens as well as the unique needs of the AYA population.
7.3.2 The “Adult” Approach
There have been essentially two approaches by medical oncologists to the treatment of ALL in adults. One approach is administration of BFM-like chemotherapy, as outlined above. The other is the “hyper-CVAD” regimen, which consists of cycles composed of two alternating courses of chemotherapy (course A and course B) [36]. Course A consists of cyclophosphamide and mesna given every 12 h on days 1–3, followed by doxorubicin on day 4. Vincristine is given on day 1, and there are 2, 4-day pulses of dexamethasone on days 1–4 and 11–14. Course B consists of intermediate-dose methotrexate on day 1 with leucovorin rescue followed by high-dose cytarabine given every 12 h on days 2–3. Methylprednisolone is administered on days 1–3. Intrathecal MTX and cytarabine and cranial radiation are used for CNS prophylaxis and incorporated into both course A and course B, the amount of which depends on a risk categorization for CNS relapse. Rituximab is added to each A and B course for patients with CD20+ ALL. After eight cycles of therapy, patients receive POMP maintenance therapy, consisting of 6MP, vincristine, MTX, and prednisone, with two courses of intensification with hyper-CVAD and MTX/asparaginase at months 6–7 and 18–19 of maintenance therapy. When treated with hyper-CVAD, patients receive each course of myelosuppressive drugs followed by several weeks of rest while awaiting count recovery. With either the BFM or the hyper-CVAD approach, the maintenance phase of therapy tends not be as prolonged as that of pediatric protocols [36–40].
7.3.3 “Pediatric” Versus “Adult” Approaches
A number of studies have compared outcomes for similarly aged AYA patients treated with either pediatric or adult regimens or have reported outcomes specifically of AYA patients treated on pediatric-inspired regimens (Table 7.1). In many of these, survival was significantly better for those patients treated with a pediatric regimen [6, 39, 41–45]. Encouraged by these results, several adult groups have prospectively applied a pediatric-inspired treatment regimen to a young adult population, managed by medical, rather than pediatric, oncologists.
Table 7.1
Outcomes for AYA ALL patients treated on pediatric compared to adult protocols
Regimens (Pediatric/Adult) | Patient Ages (Years) | Event Free Survival (95 % CI) (Pediatric/Adult) | Overall Survival (95 % CI)(Pediatric/Adult) |
|---|---|---|---|
FRALLE-93/LALA-94 [39] | 15–20 | 67 % (±13 %) / 41 % (±14 %)p < 0.0001 | 78 % (±11%) / 45 % (± 11 %)p < 0.0001 |
DCOG / HOVON [41] | 15–18 | 69 % (54–81 %) / 34 % (21–48 %)p = 0.0001 | 79 % (64–88 %) / 38 % (24–52 %)p < 0.0001 |
NOPHO-92 / Swedish Adult ALL Group [42] | 15–18/15–20 | 74 % (60–89 %) / 39 % (19–59 %)p < 0.01 | Not reported |
ALL97 / UKALLXII [43] | 15–17 | 65 % (52–78 %) / 49 % (37–61 %)p = 0.01 | 71 % (59–84 %) / 56 % (48–68 %)p = 0.04 |
CCG/CALGB [44] | 16–20 | 63 % (24–44 %) / 34 % (55–72 %)p < 0.0001 | 67 % (58–75 %) / 46 % (36–56 %)p = 0.0002 |
NOPHO/Finnish Leukemia Group [50] | 10–16/16–25 | 67 % (±5 %) / 60 % (±6 %)p = 0.25 | 77 % (±40) / 70 % (±6 %)p = 0.29 |
MD Anderson: ABFM/hyper-CVAD [36] | 13–39/16–40 | Not reported | 74 % / 71 %p = ns |
PETHEMA ALL-96* [45] | 15–30 | Adolescents: 60 % (43–77 %)Young Adults: 63 % (48–78 %) | Adolescents: 77 % (63–90 %)Young Adults: 63 % (46–80 %) |
CCG-1961* [6] | 16–21 | 71.5 % (SE 3.6 %) | 77.5 % (SE 3.3 %) |
From 2002 to 2008, DFCI investigators administered a DFCI Pediatric ALL Consortium regimen to 92 patients 18–50 years of age, reaching an 85 % complete remission (CR) rate (90 % CI, 77–91 %) and 4-year disease-free survival (DFS) rate of 69 % (95 % CI, 56–78 %) for those patients achieving CR, which was much better than historical survival rates of <50 % [46]. The regimen, which relied heavily on intensive asparaginase administration, was well tolerated in the young adult population, and in fact the primary endpoint of the study was the feasibility of weekly asparaginase administration for the 30-week intensification phase of therapy. Fifty-seven patients were evaluable for the endpoint, and 36 of them (63 %) completed all 30 doses. Forty-one (72 %) completed at least 26 doses, an important benchmark, as the pediatric DFCI data suggests that administration of at least 26 doses of asparaginase is as efficacious as administration of all 30 [47]. In a larger cooperative group trial (C10403) that was conducted from 2007 to 2012, 296 evaluable patients between the ages of 16 and 39 were treated by medical oncologists, using one arm of the most recent COG study (AALL0232) for patients with B-ALL [48]. The 2-year EFS was 66 % (95 % CI, 60–72 %) with a median EFS of 59.4 months, significantly longer than the null hypothesis, which was that the median EFS for this patient population would be ≤32 months. The observed toxicities were similar to that reported on the pediatric trial with the exception of higher rates of thrombosis, neuropathy, osteonecrosis (ON), and mucositis in patients ≥20 years of age on C10403, but higher rates of hypersensitivity and motor neuropathy on AALL0232 [49]. Taken together, these studies demonstrate that not only is pediatric therapy feasible in a young adult population but it also appears to improve outcomes.
Other retrospective studies have reported similar outcomes for patients treated on either adult or pediatric regimens. In a population-based study, Usvasalo et al. reported that consecutive patients aged 10–25 years treated in Finland for ALL from 1990 to 2004 had comparable outcomes when treated on either pediatric or adult regimens. The 5-year EFS/OS for pediatric versus adult patients were 67 % versus 60 % (EFS) and 77 % versus 70 % (OS) (p = ns for both) [50]. In a study from MD Anderson Cancer Center, 85 AYA patients (12–40 years) were treated with a BFM-based chemotherapy regimen, and outcomes were compared to a historical control group (71 patients aged 16–40 years) treated with hyper-CVAD [36]. Similar to the study out of Finland, outcomes on each regimen were comparable. Overall survival at 3 years was 74 % for patients treated with BFM-based therapy and 71 % for those treated with hyper-CVAD (p = ns).
It is worth noting that the adult regimens used in Finland tended to incorporate higher doses of MTX, epipodophyllotoxins, and anthracyclines than the pediatric protocols, but they also included high cumulative doses of vincristine, asparaginase, and corticosteroids – tenets of BFM-based therapy. Furthermore, the authors point out that all patients in Finland, regardless of age, are treated at one of five centralized academic centers, and the culture and healthcare system is such that adherence to therapy is universally very good. Likewise, the study from MD Anderson Cancer Center is a single-institution study, and the treating physicians were very familiar with the complexity and nuances of the treatment regimens.
The potential and accrued toxicity of therapy and the late adverse effects are also increasingly major considerations, especially as the long-term survival and cure rates have improved in AYAs with ALL. As a result of decades of attempts by pediatric oncologists to reduce the adverse late effects of their regimens, the pediatric regimens have low gonadotoxic, cardiotoxic, infertility, and carcinogenic potential, achieved in part by successful limitation of their total alkylating, anthracycline, and radiation exposure, manifesting as favorable long-term follow-up results [51]. In contrast, the hyper-CVAD regimen has substantially greater exposure to alkylators, anthracyclines, and radiation, and for patients with CD20+ ALL, rituximab [52]. Hyper-CVAD requires hospitalization for the A and B courses, and there is a high rate of admission for fever and neutropenia and for other signs of infection or cytopenia [52]. Secondary acute myelogenous leukemia and myelodysplasia may also occur more frequently after hyper-CVAD than with a pediatric type of regimen, which has led to discontinuation of the regimen at some centers [52]. Hyper-CVAD is also regarded to have high potential for rendering males and females infertile, with more than 80 % of women predicted to develop amenorrhea posttreatment and a majority of men predicted to have prolonged azoospermia [52].
7.3.4 The Role of Hematopoietic Stem Cell Transplant
Some studies have supported a role for hematopoietic stem cell transplant (HSCT) in first remission (CR1). In a joint trial between the MRC and the Eastern Cooperative Oncology Group (ECOG), patients aged 15–59 years were treated with two identical cycles of induction chemotherapy, followed by allogeneic HSCT if a matched sibling donor (MSD) was available, or if there was no MSD, randomized to chemotherapy alone versus autologous HSCT [53]. A donor versus no-donor analysis of Philadelphia chromosome-negative (Ph-) patients revealed a 5-year OS of 54 % compared to 44 % (p = 0.007) for patients with and without a MSD, respectively. This survival benefit was observed in patients considered to have standard-risk disease (5-year OS 63 % versus 52 %, p = 0.02), but not necessarily in high-risk patients (5-year OS 42 % versus 35 %, p = 0.2). In a meta-analysis of 20 studies in which adult patients with ALL who had a MSD underwent allogeneic HSCT, and those who did not underwent either autologous HSCT or were treated with chemotherapy alone, there was no benefit found for autologous HSCT [54]. Overall survival was longer for those patients with a donor (OR = 0.87, 95 % CI 0.79–0.96, p = 0.006), despite higher treatment-related mortality (TRM) (OR = 2.36, 95 % CI 1.94–2.86, p < 0.00001). TRM was particularly high in patients ≥35 years of age, with 32 % of those with a donor and 14 % of those without a donor dying in remission. In patients <35 years of age, 19 % of those with and 8 % of those without a donor died without relapse. This led to higher 5-year survival rates for patients <35 years of age with a donor (55 % versus 45.1 %), but not for those ≥35 years of age (39.2 % versus 37.2 %).
Results such as these have led many oncologists to consider HSCT in CR1 for young adult patients with ALL, who are able to tolerate the toxicity of HSCT better than their older adult counterparts [55]. However, a benefit for HSCT in CR1 is not necessarily evident. A study from the International Bone Marrow Transplant Registry (IBMTR) compared outcomes between 422 patients aged 18–50 years who underwent related or unrelated HSCT and 108 patients aged 18–50 years who were treated with chemotherapy alone, according to a pediatric regimen [56]. Cumulative incidence of relapse was similar between the groups, but TRM was significantly lower in patients treated with chemotherapy alone. Disease-free survival at 4 years was significantly higher in the cohort treated with chemotherapy (71 % [60–79 %] versus 40 % [35–45 %], p < 0.0001), as was 4-year OS (73 % [63–81 %] versus 45 % [40–50 %], p < 0.0001). In a large study conducted by the Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL), investigators evaluated the role of HSCT in adults (15–55 years) with Ph- ALL who were treated with a pediatric-inspired regimen [57]. Only patients who were considered to have high-risk disease based on a combination of cytogenetics/disease biology, CNS status, and disease response were eligible for HSCT; out of 522 study patients, 282 were in the HSCT cohort. For patients who underwent HSCT, there was no benefit regarding relapse-free survival (RFS) (hazard ratio [HR], 0.80; 95 % CI, 0.6–1.06; p = 0.12) or OS (HR, 0.76; 95 % CI, 0.57–1.02; p = 0.069). There was a lower cumulative incidence of relapse in the HSCT group (HR, 0.5; 95 % CI, 0.35–0.7; p < 0.001), but this was countered by higher rates of non-relapse mortality (HR 1.46; 95 % CI, 1.09–1.95; p = 0.011), leading to the similar survival outcomes. Note, however, that on subgroup analysis, there appeared to be a benefit with HSCT for patients with poor disease response, defined as residual disease ≥10−3 after induction chemotherapy; HR was 0.37 for RFS (95 % CI, 0.2–0.69; p = 0.001) and 0.41 for OS (95 % CI, 0.22–0.76; p = 0.005). Patients with IKZF1 gene deletions also appeared to benefit from HSCT, but this may have been related to poor disease response, as often occurs in these patients. No randomized comparisons currently exist between continuation of intensive pediatric-inspired chemotherapy regimens and allogeneic HSCT in CR1 for AYAs with Ph- ALL, and these data suggest that there may not be a general survival benefit for HSCT in CR1 for this population. However, there may be identifiable subgroups of patients for whom HSCT in CR1 is beneficial.
7.4 Toxicity
Toxicity of therapy is certainly of concern for any patient with cancer, and the risks of treatment must be carefully balanced with the goal of cure. As pediatric-inspired regimens are administered to AYAs, the potential toxicity of therapy must be monitored closely, as the intensity of pediatric ALL therapy may not be as well tolerated in AYAs as it generally is in younger children. While many chemotherapy side effects are consistent across age groups, there are particular drugs and toxicities that warrant specific consideration in the AYA population.
7.4.1 Asparaginase
Asparaginase has long been established as an integral drug in pediatric ALL regimens [47, 58–62] and is effective for adults with ALL as well [6, 46, 63–65]. With its efficacy, however, comes not insignificant toxicity [66–69], and asparaginase toxicity is of concern as pediatric ALL protocols are being increasingly applied to the AYA population [70].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree