Organ
Clinical manifestations
Histologic findings
Alternate diagnoses
Skin
Erythematous maculopapular rash involving the palms, soles, pinnae, spreading to the trunk and later extremities. +/− pruritis. Bullae/desquamation in severe cases
Basal vacuolization, necrotic epidermal cells, lymphocytes in dermis, exocytosis in epithelium
Chemotherapy/radiation effect
Drug eruption
Viral exanthem
Infection
Liver
Hyperbilirubinemia, jaundice. Possible hepatitis with transaminitis, elevated alkaline phosphatase
Bile duct damage, bile duct lymphocytic infiltration, endothelialitis
Sinusoidal obstructive syndrome
Medication effect
Extra-hepatic obstruction
TPN
Infection
Iron overload
GI
Anorexia, nausea, vomiting, diarrhea, abdominal pain/ileus, GI bleeding
Apoptosis, crypt cell necrosis and drop out, epithelial denudation
Chemotherapy/radiation effect
GI tract infection (Clostridium difficile, CMV, etc.)
Drug reaction
1.
Skin: Classically manifests as an erythematous, maculopapular rash +/− pruritus involving the pinnae, palms, and soles. This rash often spreads to involve the neck and trunk with later involvement of the extremities. Severity is determined by the percentage of body surface area (BSA) involved (see Fig. 18.1) and may range from a mild, nonpruritic rash to bullous formation and desquamation reminiscent of toxic epidermal necrolysis.
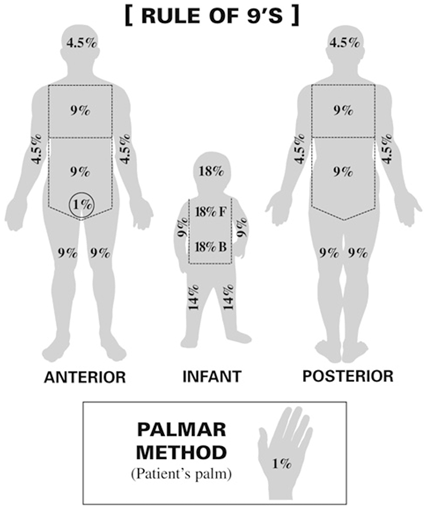

Fig. 18.1
Rule of nines (Body surface area). (In adults, the “rule of nines” can be used to determine the total percentage of area affected for each major section of the body)
2.
Liver: An elevated serum bilirubin is the typical manifestation of liver involvement, although elevated alkaline phosphatase may also be an indicator of impending disease. A variant of liver aGVHD has also been described that manifests as hepatitis with transaminitis and elevated alkaline phosphatase; however, these are not classic findings and are not specific.
3.
GI: Manifestations include anorexia , nausea, vomiting, diarrhea, and/or abdominal cramping. However, these are relatively nonspecific findings and may be attributed to the conditioning regimen, immune suppressive medications or infections.
18.5 Evaluation and Diagnosis
Tissue pathology is the gold standard for diagnosis of GVHD; however, the sensitivity of biopsy testing is ~ 60 %. Therefore, clinical correlation is necessary as many non-GVHD causes (tissue damage from the conditioning regimen, infection, medications, drug eruptions, viral exanthems) may mimic the pathologic findings of GVHD.
Efforts are under way to identify potential peripheral blood biomarkers to diagnose and guide the management of GVHD. Small retrospective studies have proposed multiple biomarkers including IL-2 and TNF-α which are markers of generalized inflammation and new lymphoid surface expression molecules such as CD30. Newer methods, including proteomics (study of complete sets of protein molecules), have identified several molecules, such as elafin, which are secreted as a result of end-organ damage and have been shown in early studies to correlate with prognosis.
1.
Skin:
a.
Dermatology consult for skin biopsy. Criteria for diagnosis of aGVHD include evidence of basal vacuolization, necrotic epidermal cells, lymphocytes in the dermis, and exocytosis in the epidermis.
2.
Liver:
a.
Liver ultrasound to rule out (r/o) sinusoidal obstructive syndrome (SOS), cholelithiasis, and/or biliary sludge.
b.
Consider liver biopsy for tissue diagnosis, either ultrasound-guided percutaneous or transjugular if patient is thrombocytopenic.
3.
GI:
a.
Stools to r/o Clostridium difficile and other enteral pathogens.
b.
GI consult for endoscopy. There is no clear correlation between endoscopic findings and aGVHD stage.
c.
To make the diagnosis of aGVHD, apoptosis must be present on pathology review. However, this finding is not exclusive to aGVHD.
i.
A small study of GI pathology identified a combination of lamina propria eosinophia (> 15/10 HPF), combined with a lack of endocrine cell aggregates and apoptotic microabscesses as indicators of mycophenolate colitis rather than gut aGVHD.
18.6 Staging/Grading
Standardized staging of aGVHD is critical to evaluating extent of disease, response to therapy and prognosis. The most widely used Glucksberg staging criteria, developed in 1974, are organ-specific and based on percentage of BSA involved, volume of diarrhea and/or total bilirubin (see Table 18.2). These stages are then evaluated together, in combination with performance status, to determine an overall grade of aGVHD (see Table 18.3).
Table 18.2
Glucksberg organ staging
Stage | Skin | Liver (bilirubin) | Gut (stool output/day) |
|---|---|---|---|
0 | No rash | < 2 mg/dL | < 500 mL/day or persistent nausea |
1 | Maculopapular rash ≤ 25 % BSA | 2–3 mg/dL | > 500 mL/day |
2 | Maculopapular rash 25–50 % BSA | 3.1–6 mg/dL | > 1000 mL/day |
3 | Generalized erythroderma | 6.1−15 mg/dL | > 1500 mL/day |
4 | Generalized erythroderma + bullous formation | > 15 mg/dL | Severe abd pain, +/− ileus, +/− bleeding |
Table 18.3
Glucksberg overall grading
Grade | Skin | Liver | Gut | ECOG performance |
|---|---|---|---|---|
I | Stages 1–2 | Stage 0 | Stage 0 | 0 |
II | Stages 1–3 | Stage 1 and/or | Stage 1 | 0–1 |
III | Stages 2–3 | Stages 2–3 and/or | Stages 2–3 | 2–3 |
IV | Stages 2–4 | Stages 2–4 and/or | Stages 2–4 | 3–4 |
There have been attempts to modify the Glucksberg system to identify a correlation of patterns of organ involvement with treatment-related morbidity and treatment failure. In 1994, following a consensus conference on aGVHD grading, the Minnesota group devised a system based on the Glucksberg criteria for organ staging, modified to include upper GI symptoms. In 1997, the Center for International Blood and Marrow Transplant Research (CIBMTR) developed a severity index (see Table 18.4) which grades GVHD based on organ involvement alone and grouping patients with similar risks of treatment-related morbidity and treatment failure.
Table 18.4
CIBMTR severity index
Skin | Liver | GI | ||||
|---|---|---|---|---|---|---|
Index | Stage (Max) | Extent of rash | Stage (Max) | Bilibrubin (µmol/L) | Stage (Max) | Diarrhea (mL/d) |
A | 1 | < 25 % | 0 | < 34 | 0 | < 500 |
B | 2 | 25–50 % or | 1–2 | 34–102 or | 1–2 | 500–1500 |
C | 3 | > 50 % or | 3 | 103–255 or | 3 | > 1500 |
D | 4 | Bullae or | 4 | > 255 or | 4 | Pain, ileus |
More recently, standard clinical findings have been evaluated in the context of newly identified biomarkers in an attempt to classify patients into low-, intermediate-, and high-risk groups. This would allow for risk stratification to better customize initial and secondary treatments, predict prognosis, and allow for more meaningful interpretation of clinical trial results due to greater homogeneity in the enrolled patient population. For patients receiving therapy on a study protocol, one should become familiar with the staging system associated with that protocol to ensure accurate and consistent measurements of aGVHD.
Patients who develop grade 1 or 2 aGVHD have an 80 % probability of long-term survival. Survivorship falls to 30 % for patients with grade 3 disease and 5 % for patients with grade 4 disease.
18.7 Treatment (See Chap. 11 for discussion of GVHD Prophylaxis)
The standard mainstay of treatment for aGVHD is corticosteroids; however, not all patients achieve durable responses to steroids alone. Two recent multicenter trials were conducted through the Bone Marrow Transplant Clinical Trials Network (BMT CTN) to evaluate initial therapeutic options for newly diagnosed GVHD .
1.
BMT CTN 0302: Phase II trial randomizing patients with newly diagnosed aGVHD between four drugs, all given in combination with steroids: etanercept, mycophenolate (mycophenolate mofetil, MMF), denileukin diftitox, and pentostatin:
a.
Efficacy, survival, and toxicity all favored MMF
b.
Approximately 50 % of patients receiving MMF did not achieve target drug levels; patients with drug levels > 0.5 mcg/mL at weeks 1 and 2 had a significantly greater proportion of complete and partial responses at days 28 and 56, suggesting an MMF dose higher than 1 gm BID as prescribed in the trial is necessary to achieve a response.
c.
These data supported further study of MMF as primary therapy.
2.
BMT CTN 0802: Phase III, double-blinded, randomized trial comparing steroids + MMF versus placebo:
a.
MMF dosing was increased to 1 gm q8 h based on data from CTN 0302.
b.
Study participation was terminated at interim analysis when no difference was observed between the two groups with regards to rates of GVHD, GVHD-free survival, overall survival, development of chronic GVHD, rate of Epstein–Barr virus (EBV) reactivation, and cumulative incidence of grade 3 infections.
c.
Benefit of adding MMF to corticosteroid therapy for new diagnosis aGVHD was not confirmed.
3.
General treatment guidelines:
a.
There is no consensus on initial corticosteroid dosing or tapering schedule:
i.
Should patient’s rash progress to > 50 % of BSA or patient develop aGVHD involving the gut or liver, systemic steroids should be dosed at 1–2 mg/kg/day depending on the current and potential predicted severity of aGVHD.
ii.
For patients with stage 1 and 2 disease, there is no evidence that beginning with 1 mg/kg/day of steroid results in worse patient outcomes overall. Additionally, no benefit has been shown with steroid doses > 2 mg/kg/day.
b.
Maximize benefit of calcineurin inhibitors (CNIs) in combination with steroids by maintaining therapeutic drug levels (cyclosporin A (CSA) ~ 200 ng/ml, tacrolimus ~8–10 ng/ml)
c.
To avoid potential side effects of protracted high-dose steroids, tapering should begin after 7 days of therapy regardless of response:
i.
There are no clear guidelines for steroid tapering.
ii.
One could consider a step-wise decrease by 0.25 mg/kg/day every 5–7 days to a dose of 1 mg/kg/day, then continue to decrease by 10 % every 7 days as tolerated.
d.
The most important predictor of long-term survival is response to high-dose steroids.
i.
Response at day 28 of therapy is considered to be the best predictor of 2-year transplant-related mortality (TRM):
A study from Minnesota evaluated the response of 864 patients with aGVHD to high-dose steroids (60 mg/m2/day):
◦ Complete response rate was 53 % and complete/partial response rate of 65 % at day 28.
◦ Additional data suggested that most patients who would respond to therapy would do so by day 28.
◦ Patients with no response by day 28 were 2.78 times more likely to experience TRM at 2 years than those who responded to therapy.
ii.
Due to infection and organ failure, steroid refractory disease is associated with a high rate of morbidity and mortality.
e.
Ensure adequate antifungal and antiviral prophylactics are in place (see Chap. 10 for monitoring and prophylaxis guidelines). Change to intravenous (IV) formulation if absorption is questionable due to diarrhea:
i.
Acyclovir 800 mg po BID or 250 mg/m2 IV daily
ii.
Weekly monitoring of CMV PCRs remains critical as aGVHD often accompanies CMV reactivation
iii.
Maximize fungal coverage:
Posaconazole (Noxifil®) 200 mg po TID (suspension) or 300 mg po daily (tablet); however, therapeutic drug levels may be difficult to achieve in patients with GI aGVHD due to absorption issues
Voriconazole (VFend®) 4 mg/kg po/IV BID
If patient is unable to tolerate azoles due to transaminitis, consider low-dose liposomal amphotericin 1 mg/kg IV daily or 3 mg/kg IV three times weekly
f.
Consider surveillance for EBV, adenovirus and human herpes virus 6 due to profound T cell suppression associated with GVHD therapy.
4.
Organ specific:
a.
Skin:
i.
Stage 1 and 2 skin GVHD can be treated with topical steroids such as triamcinolone 0.1 % or betamethasone 0.1 % cream or ointment. These moderate-dose topical steroids should be used only on the trunk and extremities. Hydrocortisone 1 % is safe for application to the face, neck, and groin. If possible, wrap affected areas after application to provide occlusion to increase absorption.
ii.
Emollients to prevent breakdown of dry and fissured skin areas.
iii.
Keep skin clean and dry, using gentle hypoallergenic soaps.
iv.
Antipruritic agents (diphenhydramine 12.5–50 mg po q6 h, hydroxyzine 25 mg po QID)
b.
Liver:
i.
Hold medications which may contribute to hyperbilirubinemia (particularly azoles)
ii.
Consider ursodeoxycholic acid (ursodiol, Actigall®) 300 mg po BID to increase water solubility of bile salts and protect liver cells from toxic bile acids
c.
GI:
i.
Nothing by mouth (NPO) or stage I GVHD diet (see Appendix) depending on symptoms
ii.
IV hydration. Consider total parenteral nutrition (TPN) early depending on severity of symptoms
iii.
Change all immune suppression to IV formulation to ensure absorption
iv.
Supportive care with antiemetics and antidiarrheals
v.
Consider gram-negative prophylaxis or anaerobic protection in light of compromised mucosal integrity:
Ciprofloxacin (Cipro®) 500 mg po BID or 400 mg IV BID
Levofloxacin (Levaquin®) 400 mg po/IV daily
Imipenem (Primaxin®) 500 mg IV q6 h
vi.
Oral nonabsorbable steroids may be considered as an adjunct to systemic therapy
Beclomethasone (OrBEC®)
Budesonide (Entocort®)
18.8 Steroid Refractory Disease
There is no standard definition of steroid refractory aGVHD. However, failure of therapy has been defined as progression of symptoms after 3 days of high-dose steroids or no improvement after 7 days of therapy. Approximately 40 % of sibling-donor and 25 % of unrelated-donor transplant patients will respond to therapy; 60–75 % of patients will require additional therapy. The addition of second-line therapy is associated with a 1-year survival rate of 20–30 %.
There is also no consensus on the best salvage therapy for steroid refractory disease. Multiple agents have been utilized with varying degrees of success. However, in the past 30 years, no products have been approved by the Food and Drug Administration for the systemic treatment of aGVHD. The choice of second-line therapy should be based on the effects of prior treatment, potential for drug interactions, toxicity profile, and provider/patient preference (see Table 18.5):
Table 18.5
Agents for salvage therapy in steroid refractory GVHD
Drug | Class | Dose/route | Preferred use | Current FDA approval |
|---|---|---|---|---|
Alemtuzumab (Campath®) | MAB | 10 mg IV/day × 5 doses | Skin, liver | B cell CLL |
ATG—equine (ATGAM®) | Immune serum | No defined standard dosing | Skin, GI, liver | Aplastic anemia; prevention/treatment of renal transplant rejection |
ATG—rabbit (Thymoglobulin®) | Immune suppressant | 2.5 mg/kg IV × 4–6 days or 2.5 mg/kg QOD on days 1, 3, 5, and 7 | Skin, GI, liver | Renal transplant rejection |
Basiliximab (Simulect®) | MAB | No defined standard dosing | Skin | Prevention/treatment of renal transplant rejection |
Beclomethasone (orBec®) | Adrenal glucocorticoid | 2 mg po q6 h of both immediate release & enteric coated capsules | GI only | Orphan drug status |
Budesonide (Entocort®) | Adrenal glucocorticoid | 3 mg po TID or 9 mg po daily | GI only | Crohn’s disease |
Etanercept (Enbrel®) | TNF inhibitor | 25 mg SQ twice weekly × 4–8 weeks | GI | Ankylosing spondylitis, chronic plaque psoriasis, RA, juvenile idiopathic arthritis
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
