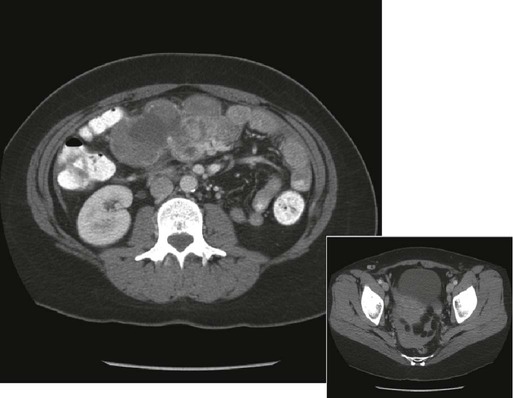
Fumito Ito and Alfred E. Chang • Perforation affects approximately 20% of patients with acute abdominal emergencies. • Bowel perforation can be due to spontaneous tumor rupture, tumor necrosis secondary to chemotherapy, radiation therapy, drugs (e.g., steroids), or inflammatory conditions. • Operative intervention is mandated unless the patient’s overall prognosis is poor. • Bleeding affects approximately 15% of patients with acute abdominal emergencies. • Bleeding is more commonly seen in patients with leukemia or lymphoma who are undergoing chemotherapy. • Endoscopy is critical to identify the source of bleeding and can even be therapeutic. • Nonoperative therapy is successful for many patients. • Also termed necrotizing enterocolitis, neutropenic enterocolitis typically affects the terminal ileum, cecum, and ascending colon in patients with chemotherapy-induced neutropenia. • Most patients respond to conservative management with broad-spectrum antibiotics and bowel rest. • Surgical intervention should be considered for perforation, uncontrolled sepsis, or persistence of symptoms despite correction of neutropenia. • Obstruction affects approximately 40% of patients with cancer who experience acute abdominal emergencies. • One fourth to one third of patients who require surgical intervention have a benign cause of their obstruction. • Partial bowel obstruction can initially be treated nonoperatively, which is successful 25% of the time. • Cross-sectional imaging gives valuable information about the location and etiology of malignant biliary obstruction and resectability of tumor. • Manifesting symptoms rarely match those of intraabdominal malignancy and more commonly represent complications after surgery or radiation therapy or both. • Medical management consisting of nutritional support and bowel rest allows spontaneous closure of most enterocutaneous fistulas. • Causes for persistence of a fistula include undrained infection, luminal obstruction distal to the fistula, prior radiation, epithelialization of the fistulous tract, cancer within the tract, presence of a foreign body, and malnutrition. The classic general surgery approach to a patient with acute abdominal pain entails identification of a potentially life-threatening problem and evaluation for emergency exploratory laparotomy. In patients with cancer, the etiology of acute abdominal pain may be directly related to a malignant process, but seemingly unrelated processes must be considered as well. Some GI cancers can manifest as abdominal emergencies that require surgical intervention. Problems that require surgical consultation include obstruction, perforation, hemorrhage, inflammatory processes, and other miscellaneous problems such as fistulae (Box 47-1).1,2 In a prospective analysis of more than 1000 consecutive palliative procedures in patients with cancer, more than 50% were performed on the GI system, with obstruction and bleeding making up more than 75% of the manifesting symptoms. The procedures were either operative (70%) or endoscopic (30%) intervention, resulting in initial symptom resolution in 80% of patients, although they were associated with significant morbidity (40%), mortality (10%), and limited anticipated survival (approximately 6 months).3 The decision to intervene with an invasive procedure is often difficult because of abnormal physiological responses to injury and inflammation, complications associated with previous cancer therapies, and competing risks due to extent or stage of the underlying cancer. It requires a multidisciplinary approach, and in some instances, medical management or use of palliative measures makes up the mainstay of therapy. Interactions among the patient, the family, and the surgeon (palliative triangle) are important to guide individual decisions regarding care. Abdominal pain is the most common symptom in the patient with an acute abdomen. In patients with a diagnosed intraabdominal malignancy who are undergoing chemotherapy and/or radiation therapy, abdominal pain must be investigated carefully. Care must be taken not to automatically attribute these complaints to cancer progression. Important considerations in the evaluation of the patient with an acute abdominal process include hemodynamic stability and deterioration of symptoms during the course of examination and workup. Patients taking corticosteroids or who have an altered sensorium warrant special attention because of a potentially unreliable physical examination. Other abdominal complaints such as vomiting, distention, lack of flatus, and fever should raise the examiner’s index of suspicion that an emergent problem is present. Ultimately, the surgeon must make the determination as to whether the patient has an acute abdominal process that requires surgical intervention. Decisions to operate on acutely ill patients with cancer are difficult and often require a high level of surgical decision making. Due consideration must be given to the stage and prognosis of the cancer itself, because the surgeon’s ability to offer a procedure with curative intent versus palliation only may alter the decision-making process. The role of palliative procedures has increased tremendously, with as many as 6% to 21% of cancer operations being classified as palliative in nature.3,4 Even without curative intent, surgeons have a great deal to offer in terms of symptom relief and overall improvement in quality of life.3 However, attempts at successful palliation must be balanced with inappropriately aggressive attempts, which may carry unacceptable rates of morbidity and mortality. Perforation of the GI tract mandates operative intervention unless the patient’s condition is judged to be extremely poor. Excluding iatrogenic causes of bowel perforation, the causes of perforation can be categorized into the following subgroups: spontaneous tumor rupture or erosion into bowel, drug-induced or associated perforations, and inflammatory conditions. GI tract cancers may present with perforation as the precipitating event, but this presentation is rare and is associated with high mortality rates. Primary colon cancers can present as localized perforations or can cause obstruction with subsequent perforation. Whether perforation is associated with poorer long-term overall survival is unclear; the perioperative mortality rate after presentation with colon cancer perforation, either at or proximal to the tumor site, remains high, ranging from 17% to 48%.5,6 Spontaneous perforation of GI tumors is more common in patients with lymphomas involving the GI tract, although this diagnosis is rare. Large malignant retroperitoneal lymphomas, renal cell carcinomas, and testicular cancers metastatic to aortocaval nodes can invade the adjacent duodenum and cause perforation. Patients with GI lymphomas can have symptoms at presentation or can experience symptoms related to regression of their primary tumor during treatment with chemotherapy and/or radiation therapy. Perforation or bleeding from treatment-induced tumor lysis occurs in approximately 3% and 5% of patients, respectively. Although resection of larger, higher grade GI lymphomas can be associated with increased morbidity, the mortality rate is greater than 50% when surgical intervention is urgently required for perforation or bleeding in the setting of neutropenia and thrombocytopenia.7,8 The true incidence of significant GI bleeding in patients with cancer is not well defined. An estimated 10% to 15% of patients with abdominal emergencies requiring operative intervention had abdominal hemorrhage, most of them resulting from intraluminal bleeding.1 Patients with lymphoma or leukemia who are treated with combination chemotherapy regimens can experience GI tract bleeding. Although a GI tumor itself could be the source of bleeding, the differential diagnosis should include non–tumor-related sources as well. The most common causes of upper GI tract bleeding in patients with cancer are peptic ulcer disease or stress ulceration and complications of anticoagulation or thrombocytopenia.2 Rates are likely to be lower in clinical practice, given the availability and increasingly widespread use of H2-receptor blockers and proton pump inhibitors. Other less common causes of bleeding include Candida esophagitis, Mallory-Weiss mucosal tears, hemorrhage from inflammatory conditions (e.g., neutropenic enterocolitis), or radiation-associated complications (e.g., arterial-enteric fistulae and radiation enteropathy). The pathogenesis of upper GI bleeding can be multifactorial. Gastritis or peptic ulcers can be associated with a variety of agents, such as aspirin, alcohol, steroids, indomethacin, or phenylbutazone. Chemotherapeutic agents can depress platelet production, as well as damaging or irritating the mucosal surfaces. Although the correlation between thrombocytopenia and bleeding is well described, it is difficult to predict which patients might experience bleeding that requires transfusion or life-threatening hemorrhage. Overall, the incidence of bleeding is low, with one group reporting bleeding episodes with 9% of chemotherapy cycles.9 The vast majority of episodes were mild (e.g., epistaxis), but major hemorrhage (including GI bleeding) was seen in approximately 3% of cycles. In that study, administration of certain chemotherapy agents (e.g., cisplatin, carboplatin, carmustine, and lomustine) was noted to be significantly related to bleeding episodes. Other investigators have more recently reported thrombocytopenia, hemorrhage, and hemolysis with oxaliplatin.10 Prophylactic platelet transfusions should be considered on a case-by-case basis, especially if bleeding has occurred previously. Hepatic arterial infusion of fluorodeoxyuridine using an implanted pump for the treatment of liver tumors has been reported to result in a significant incidence of upper GI toxicity with biliary sclerosis, gastritis, peptic ulcers, and intraabdominal bleeding due to catheter displacement.11 Other unusual causes of bleeding include hemobilia secondary to the presence and/or treatment of hepatobiliary tumors. Arterial-enteric fistulization may result in severe intraluminal hemorrhage, and operative management should be approached with joint vascular surgery consultation. Communication with vascular structures should always be considered in previous surgical sites and areas of prior radiation treatment. Intraabdominal hemorrhage is usually seen as a result of tumor rupture (Fig. 47-1), but spontaneous splenic rupture in patients with hematologic malignancies can be seen as well. Rapid tumor necrosis with subsequent hemorrhage has been reported during chemotherapy and is most commonly seen in patients with GI lymphomas. The incidence of significant GI bleeding in this patient population as a result of tumor lysis from therapy is approximately 5%.12 Surgical resection of GI lymphoma before systemic or radiation therapy could be beneficial for lesions that are amenable to resection with minimal morbidity. Special consideration should be given to patients receiving targeted molecular therapy agents. Bevacizumab (Avastin), a monoclonal antibody targeting the vascular endothelial growth factor receptor, has been shown to significantly improve overall and progression-free survival rates in patients with metastatic colorectal cancer when used with combination chemotherapy compared with chemotherapy alone.13,14 Results of these randomized trials have led to the widespread use of bevacizumab in patients with metastatic colorectal cancers and in selected patients with other solid-organ cancers. Because this drug targets tumor angiogenesis, rare but serious complications, such as bowel perforation, can occur in about 1.5% to 1.7% of patients during treatment not only for colorectal cancer but also for other malignancies that lack disease within the peritoneal cavity.13,15 Risk factors for perforation are difficult to define but include the presence of an intact primary, abdominal irradiation, nonsteroidal antiinflammatory drug use, diverticulosis, and recent endoscopy.15 Another targeted molecular agent associated with bowel perforation and GI bleeding is small-molecule tyrosine kinase inhibitors including imatinib (Gleevec) and sunitinib (Sutent). Imatinib is commonly used in the treatment of chronic myelogenous leukemia and GI stromal tumors (GISTs). Sunitinib is used for GISTs that are refractory to imatinib, advanced renal cell carcinomas, and pancreatic neuroendocrine tumors. GI bleeding or intraabdominal hemorrhage in patients with GISTs has been reported to be 3% to 5% with imatinib and 3% to 9% with sunitinib.16–20 Although it is difficult to determine whether an emergency event is associated with the treatment effect or disease progression, one report showed high mortality associated with emergency surgery.20 Close follow-up would be recommended, especially for patients with necrotic or cystic degeneration shown on imaging studies during treatment. With the expanding indications for use of immunotherapeutic agents such as interleukin-2 (IL-2) for metastatic melanoma and renal cell cancer, clinicians should be alert to occasional cases of colonic infarction and perforation.21 The causes of these perforations are unknown, but it has been postulated that they arise from impaired perfusion due to hypotension, tissue edema, and vasoconstriction as a result of the use of pressor agents. A recent report suggests that bowel perforation might be more commonly seen when IL-2 is used in combination with anticytotoxic T-lymphocyte antigen 4 antibody, and that association can be seen in conjunction with autoimmune colitis.22 Bowel necrosis with perforation has been described with the use of cytosine arabinoside. In 50 patients treated for leukemia, 7 patients were found to have bowel necrosis and peritonitis at autopsy.23 Paclitaxel (Taxol), which is commonly used to treat patients with ovarian, breast, and non–small cell lung cancers, has also been reported as a chemotherapeutic agent that can cause bowel perforation unrelated to tumor lysis.24 In patients with advanced ovarian cancer, this complication is rare but serious, and its presentation is associated with a 43% mortality rate.25 Noncytotoxic drugs are the presumed cause of perforation when bowel wall injury cannot be associated with tumor necrosis or other specific factors in patients with cancer who are undergoing drug therapy. Immunosuppressed patients have a blunted inflammatory response and may have a more subtle presentation of GI perforation. The drugs that are most commonly implicated are corticosteroids, which can give rise to ulcers and perforations in various portions of the GI tract. Increasingly, aggressive chemotherapeutic regimens are being used in the treatment of patients with cancer. These treatments can expose patients to life-threatening complications related to bone marrow suppression and neutropenia. The incidence of acute illnesses necessitating surgical intervention in the setting of the neutropenic patient with cancer is approximately 7%.26 Unique inflammatory abdominal problems in patients with cancer who are receiving aggressive therapies are reviewed in this section. Neutropenic enterocolitis is a clinicopathological syndrome that involves the GI tract of patients receiving chemotherapy for hematologic and solid malignancies. The clinical condition has been given a variety of names in the past, including typhlitis, ileocecal syndrome, and necrotizing enteropathy. Most commonly seen in pediatric patients who are undergoing treatment for leukemia, neutropenic enterocolitis is still a relatively rare occurrence in adults, with an incidence of 5% in this patient population.27
Acute Abdomen, Bowel/Biliary Obstruction, and Fistula
Acute Abdomen: General Considerations
Gastrointestinal Perforation
Gastrointestinal Bleeding
Adverse Events with Cytotoxic Agents Leading to Bleeding or Perforation
Inflammatory Conditions in the Patient with Cancer
Neutropenic Enterocolitis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Acute Abdomen, Bowel/Biliary Obstruction, and Fistula