Fig. 5.1
Nodal staging using EBUS-TBNA combined with EUS-(B)-FNA. A CP-EBUS was used for EBUS-TBNA, which evaluated station 4L. The same bronchoscope was then introduced into the esophagus, and station 5 was evaluated by EUS-(B)-FNA. There were no malignant cells in the mediastinal lymph nodes, and the patient underwent radical surgery
Studies have demonstrated the cost benefits of EBUS-TBNA over mediastinoscopy for the diagnosis of patients with isolated mediastinal lymphadenopathy [14]. The ASTER trial made a similar observation [15]. In 2013, the third edition of the American College of Chest Physician (ACCP) guidelines for the management of patients with lung cancer was published [12]. The new guidelines for staging the mediastinum now regard endoscopic ultrasound staging procedures, including EBUS-TBNA and EUS-FNA, the best first-line tests, better than surgical staging for radiologically suspect lymph nodes that are accessible by endoscopy [12]. However, the guidelines also mentioned concerns about the quality of endoscopic staging and the necessity of surgical staging for patients whose lymph nodes might have a high probability of metastatic disease but were found to be negative on EBUS and/or EUS [12]. Similar guidelines were also published by European societies, which recommend ultrasound-guided nodal staging as a combination of EBUS-TBNA and EUS-FNA [16]. They recommend that endoscopists should be trained for both EBUS and EUS, so that complete endoscopic nodal staging can be performed in a single session [16]. In addition, continuous education and training is mandatory for maintaining a high diagnostic yield and maximum safety during mediastinal staging by EBUS-TBNA and/or EUS-FNA [17]. Recently, guidelines that focus on the technical aspects of EBUS-TBNA were published, and they will greatly facilitate the standardization of EBUS-TBNA procedures [18].
5.2.2 N1 Staging Using EBUS-TBNA
The several advantages of EBUS-TBNA include minimal invasiveness, repeatability, and ability to easily perform hilar lymph node sampling. EBUS-TBNA can assess the lymph nodes and lesions adjacent to the airway and within the reach of the EBUS scope. EBUS-TBNA can assess the N1 nodes in addition to the N2/N3 nodes and accurately differentiate between N0 and N1 stages [19]. The diagnosis of N1 disease may sometimes affect surgical planning prior to surgery (Fig. 5.2). Another application of EBUS-TBNA being increasingly used is the staging of mediastinal and hilar lymph nodes for patients who will undergo stereotactic radiation therapy (SBRT) [20]. Patients who are being considered for SBRT often have comorbidities that preclude them from undergoing surgical staging. However, in contrast to surgery, the existence of N1 disease is a contraindication for SBRT. A clinical trial looking at the role of EBUS-TBNA in mediastinal and hilar staging for patients scheduled for SBRT is under way (NCT01786590).
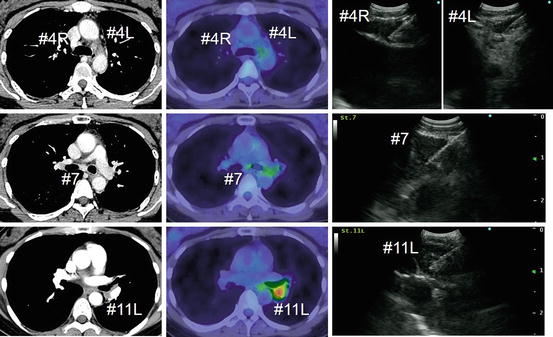

Fig. 5.2
Preoperative N1 staging by EBUS-TBNA. The patient had adenocarcinoma in the left lower pulmonary lobe. Nodal staging by EBUS-TBNA revealed malignant cells only in the station 11L lymph node (N1 disease). The patient underwent left lower lobectomy with lingular segmentectomy, and complete resection (R0) was achieved
N1 staging is also important for the treatment of small cell lung cancer. Surgery is only considered for stage I disease; therefore, the hilar lymph nodes should be carefully evaluated. EBUS-TBNA staging of small cell lung cancer has obtained a high diagnostic yield, with a sensitivity, specificity, and diagnostic accuracy of 96.4 %, 100 %, and 97.2 %, respectively [21]. Patients who have undergone surgery for N0 small cell lung cancer after nodal staging by EBUS-TBNA have had a favorable outcome [21]. Currently, the accessibility of interlobar/lobar lymph nodes to CP-EBUS is limited because of the size and angle of the scope. A novel thin EBUS scope (prototype thin convex-probe EBUS; BF-Y0046, Olympus, Japan: Fig. 5.3) is under development to improve the ability of the scope to access the distal lymph nodes [22].


Fig. 5.3
Conventional CP-EBUS and a prototype thin CP-EBUS. The prototype thin CP-EBUS is 1 mm thinner than a conventional bronchoscope
5.2.3 EBUS-TBNA for the Restaging of Patients with Lung Cancer
Mediastinoscopy has been regarded as the “gold standard” for the mediastinal staging of patients with lung cancer, because of its high diagnostic yield and safety [23]. However, the restaging of the mediastinum by mediastinoscopy (remediastinoscopy) after induction therapy is technically difficult because of mediastinal adhesions [24]. Selected patients who undergo induction therapy (chemotherapy and/or radiotherapy) before undergoing surgery require accurate restaging of the mediastinum before the resection is performed. The diagnostic yield of remediastinoscopy is limited, compared with the initial mediastinoscopy [24]. By contrast, EBUS-TBNA is an easily repeatable procedure, and EBUS-TBNA for the restaging of the mediastinum has been reported to be useful [25]. Although the diagnostic yield of restaging EBUS-TBNA is also reduced compared to an initial evaluation; EBUS-TBNA is an acceptable minimally invasive modality [26, 27]. However, most of the data on the diagnostic yield of restaging EBUS-TBNA has been retrospective. Additional prospective studies are warranted.
EBUS-TBNA has been found to be useful for assessing mediastinal lymphadenopathy in patients with previously treated lung cancer. When CT and/or PET show abnormalities within the mediastinum or the hilum, i.e., enlarged lymph nodes or 18F-fluorodeoxyglucose (FDG)-avid lymph nodes, histological confirmation is often needed to rule out reactive adenopathy vs recurrence, since radiological findings alone are not reliable [28]. In our experience, EBUS-TBNA of radiologically abnormal mediastinal lymph nodes has confirmed histological features different from those of the nodes sampled before treatment. Those findings were helpful for deciding on subsequent therapy [29], especially for the patients who were treated by epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors and developed resistance that was sometimes associated with the transformation to small cell lung cancer (Fig. 5.4) [30]. Therefore, rebiopsy and tissue confirmation is important in the management of previously treated lung cancer.
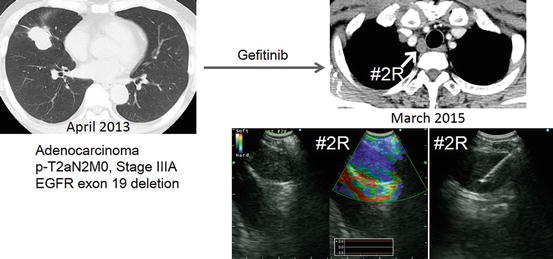

Fig. 5.4
Transformation to small cell lung cancer during EGFR-TKI treatment. The patient was administered gefitinib as adjuvant chemotherapy in a clinical trial setting. An enlarged station 2R lymph node was evaluated by EBUS-TBNA, and the lymph node was histologically diagnosed as small cell lung cancer with the use of immunostaining. The EGFR exon 19 deletion was also detected in the rebiopsied sample
5.2.4 EBUS Image Analysis for Distinguishing Between Benign and Malignant Lymph Nodes
The indication for invasive mediastinal staging in lung cancer depends on radiologic findings, which include CT and PET. In general, ultrasound can provide a more detailed high-resolution evaluation of nodal staging than CT or PET. Because of the resolution of ultrasound, there have been several important studies that have evaluated the EBUS image analysis of lymph nodes. The first study report was on the B-mode image classification of mediastinal and hilar lymph nodes using the first-generation EBUS ultrasound processor (EU-C2000, Olympus, Tokyo). B-mode images were classified according to six indicators, including size, shape (oval or round), margin (indistinct or distinct), echogenicity (homogeneous or heterogeneous), presence of central hilar structure, and presence of central necrosis sign [31]. Four predictors, round shape, distinct margin, heterogeneous echogenicity, and presence of coagulation necrosis sign, were identified as independent predictors for nodal metastasis [32]. A study that used a second-generation EBUS ultrasound processor (EU-ME1; Olympus, Tokyo) with the capability of Doppler mode evaluation evaluated vascular patterns within the lymph nodes as predictors of nodal disease [32]. This study categorized four vascular patterns and determined whether or not bronchial arterial flow was present. The use of vascular pattern classifications obtained a diagnostic yield for predicting positive or negative nodal metastasis of about 85 % [32]. Subsequent studies using B-mode classification found that EBUS was effective for differentiating metastatic vs normal lymph nodes [33–35].
The most recent ultrasound processor is equipped with an additional imaging feature, elastography, which is a strain imaging technique that assesses tissue stiffness and visualizes the distribution of stiffness in the region of interest. Malignant tissues tend to be stiffer than normal tissues because of the increased density of tumor cells and vascular structures (Fig. 5.5). Izumo et al. subjectively classified EBUS elastographic images into three categories: Type 1, predominantly non-blue; Type 2, partly blue; and Type 3, predominantly blue. They classified Type 3 as malignant and reported that 94.6 % of Type 3 lymph nodes were positive for metastasis [36]. However, for appropriate subjective categorization and classification of ultrasound image characteristics, bronchoscopists must have sufficient knowledge and experience with EBUS image analysis. We recently reported the utility of the “stiff area ratio” measured by EBUS elastography [37]. The “stiff area ratio” is a method of objective evaluation and should be more helpful in guiding bronchoscopists during selective sampling of a suspicious station or lymph node within the same station.
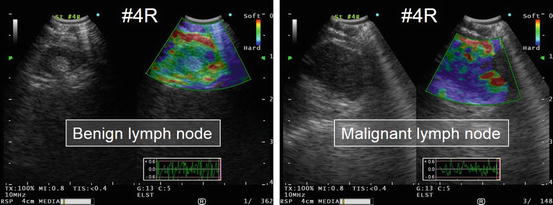

Fig. 5.5
Representative elastography images of benign and malignant lymph nodes. A benign lymph node appears colored green to yellow/red on the monitor. By contrast, a malignant lymph node appears blue, which indicates relative hardness compared with the surrounding tissue
5.3 Multidirectional Analysis Using Samples Obtained by EBUS-TBNA
5.3.1 Acquisition and Preparation of EBUS-TBNA Samples
EBUS-TBNA samples undergoing immunohistochemical analysis in addition to conventional histomorphological evaluations have led to higher rates of identifying the histological subtypes of non-small cell lung cancer (NSCLC) specimens [38]. However, the quantity of material that can be obtained by needle biopsy is small, and therefore attempts have been made to improve the processing methods used for to EBUS-TBNA samples to obtain a pathological diagnosis [39]. A needle biopsy specimen is fundamentally cytological material, and “proper preconditioning” is important for maximizing the information derived from a very small sample. However, although the effectiveness of rapid on-site evaluation (ROSE) for improving the diagnostic yield of EBUS-TBNA is still controversial, ROSE may aid in deciding how to process the sample for additional evaluations [40]. In addition, ROSE was found to increase the chances of performing successful lung cancer genotyping from small numbers of needle biopsy samples that were obtained by EBUS-TBNA [41]. The recent guidelines on the techniques of EBUS-TBNA recommended obtaining samples for histopathological diagnosis and additional samples for molecular testing [18]. The cell block method [42] and the “tissue coagulation clot” method [43] have allowed histological evaluations and have been reported to improve the diagnostic yield of EBUS-TBNA. These “core” building techniques may facilitate biomarker testing in lung cancer [44]. The World Association for Bronchology and Interventional Pulmonology recently published guidelines on the acquisition and preparation of needle aspiration samples. The guidelines also encourage bronchoscopists to have discussions with their pathologist colleagues about the most suitable methods for processing specimens [45] (Fig. 5.6).


Fig. 5.6
Specimen handling at Chiba University Hospital. First, the sample obtained by EBUS-TBNA was pushed out of the needle by a stylet, and the “core” material was processed by the tissue coagulum clot cell block method or another cell block method. Then the needle was flushed with air, and the material in the expelled air was sprayed onto glass slides. And last, the needle was rinsed with normal saline. Molecular testing of samples at each step could be performed
5.3.2 Detection of Oncogenic Drivers Using EBUS-TBNA Samples
Specimens obtained by EBUS-TBNA can be processed for multidirectional analysis [39]. Gefitinib, the first epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI), was introduced for the treatment of lung cancer in 2002 [46]. It marked the beginning of molecularly targeted therapy for lung cancer. The association between EGFR gene mutations and sensitivity to gefitinib was reported in 2004 [47, 48]. Since then, clinical molecular testing for the detection of molecular targets has increasingly been required. Because the majority of the lung cancer patients have advanced disease at the time of diagnosis, the development of molecular testing techniques on a very small biopsy specimen was warranted. The initial attempt to detect EGFR mutations in specimens provided by EBUS-TBNA was reported in 2007 [49]. Following improvements in molecular analysis, the detection sensitivity improved, and other investigators reported similar attempts at detecting EGFR mutations in EBUS-TBNA specimens [50, 51]. Multiplex mutation testing [52, 53] and mutation testing of the solution used to rinse the TBNA needle are used currently to increase the sensitivity of mutation detection [53]. In addition to EGFR gene mutations, the anaplastic lymphoma kinase (ALK) fusion gene was found to be a very strong oncogenic driver in 2007 [54]. The detection of the aberrant fusion gene will initially require immunohistochemical techniques and reverse transcription polymerase chain reaction (RT-PCR) or FISH. Sakairi et al. was able to detect the EML4-ALK fusion gene in an EBUS-TBNA sample using RT-PCR, with the result confirmed by FISH [55]. Biomarker testing of biopsy material is important for identifying which molecularly targeted therapeutic agents are useful for the patient with NSCLC. Testing should be performed prior to the start of treatment [56]. Patients with lung cancer who received the appropriate targeted therapy were reported to obtain improved survival compared with patients not receiving targeted therapy or with no oncogenic driver targets [57]. The use of cell block preparations of EBUS-TBNA specimens for molecular testing has obtained excellent results for lung adenocarcinoma; EBUS-TBNA specimens from 93 % of patients were sufficient for at least one round of molecular testing for EGFR mutations, the ALK fusion gene, and KRAS mutations [58].
5.3.3 Rebiopsy and Repeat Molecular Testing by EBUS-TBNA
The repeatable nature of EBUS-TBNA should be a powerful tool for identifying the appropriate molecularly targeted therapeutic agents. Gefitinib initially showed dramatic effects in patients with lung cancers harboring sensitive EGFR mutations; however, patients develop resistance to EGFR-TKIs. The well-known secondary changes which result in resistance to EGFR-TKIs include the substitution of methionine for threonine at position 790 (T790 M) in exon 20, which was reported in 2005 [59] and focal amplification of the MET proto-oncogene, which was reported in 2007 [60]. The secondary changes leading to resistance to ALK-TKIs include a mutation in the ATP-binding domain (L1196 M) and a mutation in the non-ATP-binding domain (C1156Y), which were reported in 2010 [61]. In addition, many other mechanisms have been reported to be involved in the development of ALK-TKI resistance [62]. Second- and third-generation TKIs have been developed to overcome various types of resistance [62]. Actually, the new-generation drugs have shown dramatic benefits for the patients with acquired resistance to initial TKI treatment [66, 64]. A previous analysis of tumor specimens taken at the time of acquired resistance to EGFR-TKI found secondary mutations, aberrant gene amplification, and transformation to small cell lung cancer [65]. The European Society for Medical Oncology (ESMO) guidelines have stated that the emergence of molecular resistance suggests that a repeat biopsy should be performed at the time of tumor progression [66]. Rebiopsy by EBUS-TBNA has been thought to be feasible [67] (Fig. 5.7); however, rebiopsy itself has so far not been a standard clinical practice because of patient factors (tolerability), physician preference, and limited resources [68].
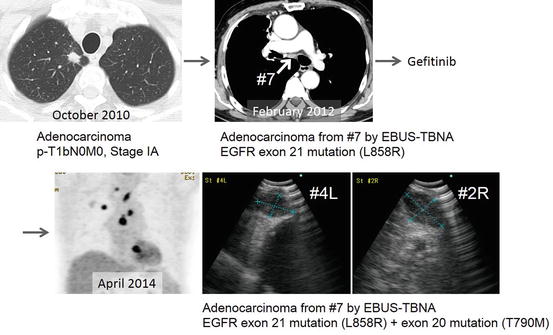

Fig. 5.7
Rebiopsy and repeat molecular testing. The patient first underwent EBUS-TBNA for the diagnosis of nodal recurrence in station 7. An EGFR mutation in exon 21 mutation was detected, and gefitinib was prescribed. Progression of disease was observed in the mediastinum, and a rebiopsy of station 4L and 2R was performed. An EGFR mutation in exon 20 mutation was detected, and the patient received cytotoxic chemotherapy
5.3.4 Application of EBUS-TBNA Samples to Molecular Analysis
Multidirectional analysis of samples obtained by EBUS-TBNA has allowed assessment of oncogenic drivers in clinics, as well as the identification of genetic signatures for lung cancer research. We can extract DNA, RNA, and protein from EBUS-TBNA samples, and these materials can be used for transcriptome and proteome analysis. Analysis of specimens with aberrant DNA methylation can be used for assessment of chemosensitivity [69] and also used for making higher sensitivity for the detection of nodal metastasis by EBUS-TBNA [70]. Expression of the unique vascular endothelial growth factor-c (VEGF-C) mRNA was reported from EBUS-TBNA samples of metastatic lymph nodes. The higher expression of VEGF-C was observed in metastatic lymph nodes with high vascularity features using Doppler mode image [71]. High-quality EBUS-TBNA samples can be used for comprehensive mRNA and microRNA expression analysis using microarray technology [72]. Primary xenograft technology using EBUS-TBNA samples has been evaluated for overcoming the limitations in sample size [73, 74].
We often encounter difficulties obtaining tissue samples, since many patients have advanced disease at the time of first presentation and are not eligible for surgery. EBUS-TBNA can solve that problem, because it can obtain tumor samples from patients with advanced disease who are not candidates for surgery. Because it can easily and safely obtain samples that are evaluable by today’s technology, EBUS-TBNA may greatly expand the knowledge base that supports lung cancer research.
5.4 Conclusions
EBUS-TBNA is now a necessary diagnostic modality for the staging of lung cancer and for providing specimens for biomarker testing. EBUS-TBNA is a repeatable procedure that can be performed to monitor the patient after treatment. Further advances in EBUS technology as well as in the needles used for tissue sampling will likely help both bronchoscopists and lung cancer investigators acquire ideal tissue samples for analysis.
Acknowledgments
Funding: This work was supported by JSPS KAKENHI (Grant-in-Aid for Scientific Research (C)), Grant Number 26462122 (T.N.).
Conflict of Interest
Takahiro Nakajima received honoraria and lecture fees from Olympus Medical Systems for EBUS-TBNA training courses.
References
1.
2.
3.
von Bartheld MB, Dekkers OM, Szlubowski A, Eberhardt R, Herth FJ, In’t Veen JC, de Jong YP, van der Heijden EH, Tournoy KG, Claussen M, van den Blink B, Shah PL, Zoumot Z, Clementsen P, Porsbjerg C, Mauad T, Bernardi FD, van Zwet EW, Rabe KF, Annema JT (2013) Endosonography vs conventional bronchoscopy for the diagnosis of sarcoidosis: the GRANULOMA randomized clinical trial. JAMA 309:2457–2464CrossRef
4.
Rintoul RC, Skwarski KM, Murchison JT, Wallace WA, Walker WS, Penman ID (2005) Endobronchial and endoscopic ultrasound-guided real-time fine-needle aspiration for mediastinal staging. Eur Respir J 25:416–421CrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree