Healthcare system factors
Low awareness of AYA cancer in primary care providers
Arbitrary referral patterns to pediatric vs. adult medical oncology providers based on age alone
Rigid upper age limits set by pediatric hospitals
Minimal collaboration between pediatric and adult medical oncologists/hematologists
Complex and often fragmented systems
Lack of relevant clinical trials (or restricted access due to age-eligibility criteria, etc.)
Inadequate access to psychology, social work, and other support services
Poor understanding of normal adolescent development and behavior
Limited research into the unique tumor biology in AYAs
Inadequate transition, survivorship, and late-effects programs
Patient-related factors
Low awareness of AYA cancers and symptoms/signs
Lack of a regular, identified primary care provider
Normal adolescent developmental traits, e.g., fixation on the present, sense of immortality, limited self-management skills
Poor self-advocacy and variable parental involvement
Limited awareness of healthcare choices
Limited ability to navigate the healthcare system
Social concerns (e.g., education, job, peers, family) outweighing health concerns
Risk-taking and adherence issues
Geographic and societal factors
Rural or remote location
Lack of insurance
Financial insecurity
Cultural and race-related inequities
The above definition of access assumes that we know the “best possible health outcome.” While survival is the most objective health outcome, it should not be forgotten that other outcomes, such as quality of life and societal outcomes (e.g., costs incurred providing a desired level of quality of care), can and should be measured. Survivors of cancer diagnosed in adolescence and young adulthood may experience difficulties returning to work and study [3] as well as a higher incidence of chronic illness and psychological morbidity [2]. Event-free survival (EFS) estimates from large clinical trials are often held as the gold standard, but as there are few AYA-specific trials, and even fewer incorporating patient-reported outcomes and health economic evaluations, an estimate of best possible outcome is difficult to determine in this group.
Even if we focus on survival alone, “real-world” survival data from population or large registry studies may not always reflect “best possible” survival outcomes. The historical example of acute lymphoblastic leukemia (ALL) is useful. Surveillance, Epidemiology, and End Results (SEER) registry data continue to show a decline in survival from ALL by age; the 2000–2004 5-year overall survival (OS) was 81 % for 10- to 14-year-olds, 61 % for 15- to 19-year-olds, 45 % for 20- to 29-year-olds, and 34 % for 30- to 44-year-olds [4, 5]. These population level data suggested that biological factors prevented AYAs from achieving better outcomes. However, Stock et al.’s seminal analysis comparing outcomes of AYAs with ALL treated on pediatric or adult studies from 1998 to 2001 challenged this assumption [6]. They found that 16- to 20-year-olds treated on a Children’s Cancer Group study had a 7-year EFS of 63 % (comparable to that of 10- to 14-year-olds in that time period), whereas those treated on adult trials had an EFS of 34 %. Recently, several prospective AYA ALL studies have confirmed that, by the use of pediatric-inspired treatment, the “best possible” outcomes for AYAs with ALL are an EFS and OS exceeding 60 % [7–14]. Therefore, while adolescent age may be a surrogate for biological and other factors that influence survival, the adverse prognostic impact of these factors can be overcome partially by access to appropriate care. This, in turn, shifts our definition of the “best possible” currently achievable survival outcome.
20.1.1 Structure of Health Services
In recent years, the AYA oncology community has directed considerable effort into understanding the elements of healthcare services – including provider, setting, and treatment – which are most critical to achieving the best outcome. There are elements of quality cancer care that should be universal for patients of all ages, which AYAs may not be accessing equally (e.g., clinical trial enrolment), and there may be age- or development-specific requirements that are unique to AYAs. Unfortunately, attempts to measure the optimal provider, setting, and treatment are complicated, and these variables are probably confounding. Ideally, studies would incorporate very large, disease-specific samples, adjustments by case mix, and long follow-up. Ongoing analyses from the AYA HOPE (Health Outcomes and Patient Experience) (United States), BRIGHTLIGHT (United Kingdom), and Patterns of Care and Experiences of Care for AYAs with Cancer (Australia) studies should contribute further to the literature on this topic.
20.1.1.1 Where and from Whom Should AYAs Access Care?
Historically, pediatric and adult cancer services have operated relatively independently of one another, with each developing its own idiosyncratic model of care, as discussed in Sect. 2.2 of this chapter. In many regions there are overlapping age limits allowing AYAs between age 15 and 20 years (and occasionally up to 30) to access either site. There are increasing data suggesting that the choice of healthcare setting influences outcome. Specifically, AYAs with tumors more commonly seen in childhood appear to have a survival advantage when treated in a pediatric center, while those with carcinomas and other “adult” tumors fare better when treated by adult oncologists [15, 16]. Although differences in regimens of chemotherapy appear to explain much of the variation in outcome in ALL between pediatric and adult centers, the survival benefit may relate also to pediatric oncologists’ greater familiarity with the complex protocols, centralization of care to tertiary/academic centers, and rigid adherence to prescribed dose and schedule [17]. These factors may explain the results of a retrospective review of German Ewing sarcoma trials which found a survival advantage for older AYAs treated in a pediatric center compared with adult centers, despite all patients receiving the same protocol of therapy [18]. Hence, it is likely that site of care is a proxy measure for clinical expertise in both disease-specific management and the psychosocial care of AYAs.
The importance of access to clinical expertise and centralized care is emphasized by several registry studies of AYAs with cancer which have reported that the adverse impact of age is abrogated by treatment at National Cancer Institute (NCI)-Designated Comprehensive Care Centers or Children’s Oncology Group institutions [15, 19–21] in the United States. While the majority of children with cancer received treatment in such centers, the proportion fell to approximately 38 % of 15- to 21-year-olds and a mere 10 % of 22- to 39-year-olds. This difference was particularly pronounced for older adolescents with low socioeconomic status, public or no insurance, and increased distance to care.
Site of care is also a strong determinant of access to clinical trials. While it is unclear whether participation in a cancer clinical trial is associated with improved survival at an individual level [22], there is no question that collaborative group trials have facilitated stepwise improvements in survival for many cancers at a population level and that AYAs have been historically underrepresented in such studies [23–27].
Decisions regarding site of care are determined frequently by the initial referring physician, who will often not be familiar with the issues discussed in this chapter, given the relative rarity of cancer in AYAs. Consequently, patterns of referral are often arbitrary and based primarily on the patient’s age, with the specialty of the referring physician, their years in practice, and location of training also potentially influencing this decision. While studies from Utah and Ohio found that 15- to 19-year-olds with “pediatric”-type tumors were somewhat more likely to be referred to pediatric oncologists than those with tumors more common in adults, two-thirds of patients from this age group were never referred to a pediatric oncologist. Furthermore, utilization of pediatric centers dropped with each additional year of age [28, 29]. This is despite consensus guidelines from the American Academy of Pediatrics [30] and the American Federation of Clinical Oncologic Societies [31] recommending that pediatric oncologists are the most appropriate providers for adolescent cancer patients.
Although there is broad agreement that pediatric oncologists are best placed to care for adolescents with cancer, the picture is less clear for young adults. Pediatric oncologists probably have the most clinical expertise in managing pediatric-type tumors, including ALL, rhabdomyosarcoma, Ewing sarcoma and osteosarcoma, and medulloblastoma, but pediatric hospitals are often not a developmentally appropriate location in which to treat young adults. Equally, one could argue that many adult hospitals lack the time and expertise to address the psychological and social challenges faced by this age group. The development of Teenage and Young Adult Cancer Units in the United Kingdom, and subsequent adaptations in other countries, may provide an ideal combination of disease-specific and psychosocial expertise as well as an age-appropriate environment. This is reflected in the British National Institute for Health and Care Excellence guidelines recommending that “young people (aged 16–24 years) with cancer have their diagnosis, treatment and support agreed and delivered by a cancer-site-specific multidisciplinary team and a young adult multidisciplinary team” [32]. The Clinical Oncology Society of Australia has released similar guidelines [33].
Extrapolating from the evidence presented above, care by physicians with expertise in AYA oncology may improve outcomes, but as yet there is little empiric evidence to support this hypothesis. The concept of what constitutes “expertise” in AYA oncology is still evolving but could be assessed by specific training (pediatric, medical, or AYA oncology), practice volume (number of AYAs seen at a center or by a provider), expertise in the cancer diagnoses that affect AYAs (regardless of patient age), and expertise in the psychosocial and developmental needs of AYAs [34, 35]. Preferably, AYA patients should have care from clinicians possessing both age-appropriate and tumor-specific expertise.
AYA oncology training programs are continuing to be developed in medical, social work, mental health, and nursing disciplines. Online and conference AYA educational opportunities to earn academic credits or continuing medical education points are becoming increasingly available for all providers (e.g., LIVESTRONG’s “Focus Under 40”), and online courses are offered internationally through Coventry University and the University of Melbourne. For physicians in the United States, a very limited number of “AYA fellowship” programs have been implemented, but are not yet recognized by credentialing boards. Until there is AYA-specific training and credentialing, the debate whether pediatric or adult medical oncologists are better placed to care for AYAs will probably continue.
Much of this discussion has focused on optimizing access to the best possible medical treatment, but AYAs with cancer also have unique psychological and social needs related to their life stage which are not always well met by either pediatric or adult models of care. As such, access to age-appropriate psychosocial care is crucial for this age group, with the critical elements summarized by Zebrack et al. [36] (Table 20.2). Anecdotal reports suggest that young people, their families, and the treating team greatly value the involvement of designated AYA cancer care coordinators (with a nursing or social work background). These health professionals can guide the patient through the often fragmented healthcare system, ensure that psychosocial issues are being addressed, help advocate for the patient, and provide general adolescent health guidance (e.g., sexual health, substance use). This is particularly important for the majority of AYAs who are treated at non-pediatric institutions, where a culture of holistic care is less engrained and self-management skills are often presumed. Early referral to psychologists and/or social workers with experience in treating AYAs is likely to help alleviate distress, promote coping strategies, and possibly support adherence to treatment. Access to other allied health staff such as an educational/vocational counselor, exercise physiologist, and dietician may assist in the transition to healthy survivorship, and it is acknowledged increasingly that exploring fertility preservation options is a fundamental component of AYA cancer care [36, 37]. AYAs with cancer endorse the benefits of meeting other young people with cancer universally [38], emphasizing the importance of facilitating access to peer support programs coordinated by not-for-profit organizations, such as Stupid Cancer (United States), CanTeen (Australia), and Teenage Cancer Trust (United Kingdom).
Table 20.2
Critical elements of quality cancer care for AYAs with cancer
Early detection and diagnosis |
Timely referral, initiation of treatment, and adherence |
Healthcare providers knowledgeable of biomedical and psychosocial issues specific to AYAs with cancer |
Supportive care and palliative care |
Clinical trials and AYA oncology research |
Fertility preservation and counseling |
Cognizance among providers of the unique psychosocial context for AYA growth and development |
Assessment and attention to cognitive, psychiatric, and psychosocial needs of AYA patients |
Referral to available age-appropriate educational, peer support, financial, and legal resources |
Facilitation of transition to survivorship |
20.1.1.2 Delivery of Therapies Which Provide Optimal Outcomes for AYAs
Research in this area is sparse, largely because of the lack of consensus on the most appropriate therapy for specific cancers in the AYA population. Many studies document variation by age in the treatment of cancer; for example, AYA sarcoma patients characteristically receive less intensive therapy than children [39–41], younger breast cancer patients are more likely to undergo mastectomy [42], and younger rectal cancer patients receive radiation more frequently [43]. As noted above, there is consensus that a pediatric-based treatment is the appropriate therapy for a young AYA with ALL; however, there is less agreement on whether this pertains to AYAs aged 30–40 as well.
The AYA HOPE Study Collaborative Group reported recently that 25 % of a population-based sample of AYA did not receive “appropriate” initial courses of treatment, as defined by expert clinical consensus [44]. In multivariate analysis, clinical trial participation and cancer type were associated significantly with appropriate treatment, with 100 % of early stage male germ cell tumor, 79 % of sarcoma, and 56 % of ALL patients receiving appropriate therapy.
This study highlights the paucity of empirical research on the appropriateness of such variation in treatment. In some clinical cases, less therapy may be more appropriate, whereas in other cases, AYAs should be treated equally or even more intensively than children. Translational and clinical research should not assume that the biology of specific cancers in AYAs is the same as in other age groups, and host genetic variations influencing treatment response may also differ in AYAs with cancer [45]. For example, the incidence of Philadelphia-like ALL increases from <3 % of children to 27 % of young adults with ALL [46]. Additionally, specific germline susceptibility alleles are overrepresented in AYAs, suggesting an inherited predisposition to high-risk disease in this age group [47]. Likewise, it is plausible that tumor genetics and host polymorphisms contribute to a more aggressive disease phenotype in AYAs who develop the epithelial cancers that more characteristically occur in older individuals. Furthermore, AYAs exhibit distinct pharmacokinetic differences to children or older adults, which may contribute to age-specific variation in drug efficacy and toxicities [48]. Whether it is appropriate to differentiate therapy for AYAs by age alone or whether more emphasis should be placed on other biologic parameters that may sometimes be correlated by age, such as hormone receptor status in breast cancer, will have to be determined for each cancer type. Clearly, this area is ripe for future translational research.
20.1.2 Timely Diagnosis
When AYAs are compared with pediatric cases, there is a clear increase in time to diagnosis (TTD) by age [49–53]. There are many more studies in adults examining TTD, some including AYAs (usually aged 30–39); they do not conclude uniformly that there is a longer TTD for AYAs compared with older adults. A comprehensive study of nearly 100,000 patients over age 30 found an increase in advanced stage at presentation in young patients with colorectal, lung, and bladder cancer, but not breast, renal, melanoma, ovarian, or prostate cancer [54]. It is unclear whether increased TTD is associated with adverse outcomes in AYAs, with tumor biology probably being more relevant. Nonetheless, it may contribute to increased morbidity in occasional cases, and perceived delays may cause heightened anxiety.
Within the AYA age group specifically, little is known about the factors influencing TTD. In a Canadian study, the delay to treatment was longer for adolescents when they were treated in an adult center than at a pediatric institution (92 vs. 57 days) [55]. The NCI Patterns of Care (POC) Study of 1,358 AYA patients with ALL, lymphoma, sarcoma, and germ cell tumors found less advanced cancer, and treatment in a hospital setting shortened the time from diagnosis to treatment [55]. Health insurance status was not a determinant of TTD in the POC study, although it was highly significant in other studies [56]. A study from the MD Anderson, for example, reported that AYA patients with public insurance had a 124 day lag-time compared with 76 days for private and 32 days for self-pay patients [57].
The interval that appears most responsible for delays in AYAs is the time from first symptom to presentation to a healthcare system [58, 59], although some studies on this topic may be subject to recall bias. Research seeking to decrease patient delays could investigate the impact of having an identified primary care provider, insurance status, cancer awareness, and self-advocacy skills. Educational interventions in healthy young people appear to increase cancer awareness [60], but it is unknown whether this results in earlier presentation. Studies in older adults have found limited evidence that education results in earlier presentation [61].
Because of the rarity of a cancer diagnosis in AYAs, primary care providers may not be sufficiently aware of common cancers in this age group, thereby contributing potentially to provider-associated delays. Research is needed to determine which symptoms should alert primary care providers to consider cancer in AYAs [62], as has been done in colon and lung cancer, and to a lesser extent in pediatric cancer [63, 64]. Clearly defined and simple referral pathways to an appropriate oncologist may also limit delays. Recent efforts in the United Kingdom and Australia have attempted to raise awareness of AYA cancer in primary care providers to facilitate early detection and referral [30, 65].
20.1.3 Affordability, Geography, and Socioeconomic Barriers to Care
There is a growing body of literature demonstrating an independent association between insurance status and access to healthcare and survival in AYAs with cancer in the United States. A study of 45,777 cases of Hodgkin lymphoma in the National Cancer Database found that uninsured/Medicaid patients had a 5-year OS of 54 % compared with 87 % in cases who had private insurance, managed care, or Medicare; this association persisted after adjustment for covariates. Uninsured patients presented at a more advanced stage had higher comorbidity scores, were less likely to receive radiotherapy and start chemotherapy promptly, and were less commonly treated at academic/research centers [66]. Likewise, a SEER review of 57,981 AYAs identified that young adults aged 25 to 39 years who had Medicaid or no insurance had a 2.9 times greater risk of death for stage I/II patients and 1.7 times greater risk of death for stage III/IV patients compared with their privately insured counterparts [67]. Another SEER analysis of 20- to 40-year-olds with cancer found that any insurance coverage, including Medicaid, was associated with a decreased likelihood of presentation with metastatic disease (odds ratio 0.84), increased receipt of definitive treatment (odds ratio 1.95), and decreased death resulting from any cause (hazard ratio 0.77) [68].
Despite the risks of recurrence and late effects of treatment, many AYA cancer survivors do not access follow-up care due to cost and underinsurance. The AYA HOPE collaborative study reported that >25 % of AYA cancer survivors experienced some period without insurance in the 35 months after diagnosis [69], and those without insurance were less likely to receive cancer-related medical care after completing treatment [70]. Even if insurance status is a marker for racial or other socioeconomic factors which may contribute to worse outcomes, this marked inequity remains extremely troubling. Furthermore, the economic impact of care is often more burdensome for AYAs. A recent survey found that, compared with their peers, young adult cancer survivors had excess annual medical expenditures of $US 3170 per person and excess annual productivity losses of $US 2250 per person [71].
For many years, young adults in the United States have had the highest uninsured rate of any age group, falling in the gap between parental coverage augmented by programs designed to provide universal health insurance to children (Medicaid and the Children’s Health Insurance Plan) and the coverage supplied by a full-time secure job [72]. The Affordable Care Act has resulted in a dramatic improvement in the number of insured young adults. Firstly, in 2010, insurance plans that include dependent coverage were required to cover adult children until their 26th birthday [73].
Early on, in 2011, 39 % of young adults aged 19–29 years remained without health insurance, primarily because their parents did not have healthcare plans that they could join. This was particularly pronounced in low-income families, with 70 % of young adults from the lowest income bracket reporting an insurance gap in the preceding year. Gaps in health insurance were associated with being less likely to have a regular doctor (85 % for those insured all year, 72 % for those with a gap in insurance, 38 % for uninsured) and delaying or forgoing healthcare because of costs [74]. Gradually more young adults took advantage, and by October 2013, the uninsured rate for 19- to 25-year-olds had dropped from 34 to 27 % [75]. In 2014, modifications to the Affordable Care Act expanded eligibility for Medicaid to young people who were unable to join their parents’ plans, as well as subsidizing private health plans for low- to middle-income individuals, thereby providing near-universal coverage for AYAs. Additional millions of young adults have gained coverage this way, and a 2015 survey reported uninsured rates for 19- to 34-year-olds at 19 % [76]. While this is cause for great optimism, gaps still exist (e.g., for young adults older than age 26 years and poor individuals in non-Medicaid expansion states), and it is important that AYAs are provided with information and assistance in navigating the process to access coverage and benefits, as many survivors remain unfamiliar with the provisions of the Affordable Care Act [77].
Studies addressing the influence of socioeconomic status on AYA cancer outcomes in countries with universal healthcare have found more mixed results. In the United Kingdom, there was no association between deprivation and survival for most AYA cancers, with the exception of leukemias and carcinomas, especially colorectal and head and neck cancers. The authors postulated that a higher incidence of smoking and unhealthy lifestyle factors in deprived regions may explain the lower survival from carcinomas [78]. For Australian AYAs with cancer, socioeconomic status is associated significantly with relative survival, but not with cancer-related mortality per se [79].
Geographic isolation may also limit access to appropriate care. In an Ohio registry study, distance was associated with a greater likelihood of adolescents being treated in a non-pediatric institution [29], although a similar study from Utah reported a negligible effect [28]. In Australia, where the relatively small population is dispersed across an enormous area, geographic isolation is linked with cancer-related mortality [78], although it is unclear to what extent this relationship is explained by the greater proportion of Aboriginal Australians living in rural and remote locations. Aboriginal Australian AYAs have higher cancer-related mortality (hazard ratio 1.47, CI 1.23–1.76), and those diagnosed with germ cell tumors have nearly seven times higher excess mortality [80]. This likely reflects the myriad of social inequities contributing to worse health outcomes in this group, somewhat analogous to the disproportionately poor survival experienced by African American AYAs and other ethnic minorities in the United States.
It is important to remember that most of the world’s cancer burden is faced by developing countries, where access to care, and consequently survival, lags well behind high-income countries. As most AYAs are in the working age group, they play an important role in their countries’ economy both now and in the future, as well as frequently supporting their families [81]. As such, broader societal consequences follow deaths from curable cancers, which were either undetected, referred at an advanced stage, or treated inadequately due to poverty, lack of access to chemotherapy and supportive care, radiotherapy, surgeons, and pathology services; abandonment of treatment; or inefficient health services. Twinning programs with high-income countries and the development of safe, efficacious, and cost-effective treatment protocols are likely to provide substantial benefits [82].
20.1.4 Adolescent Behavioral Traits
Adolescence is characterized by remarkable neurocognitive maturation and social role transitions; however, some of the normal adolescent behaviors which emerge during this process can compromise access to care. Delays in presentation may relate to the adolescent’s strong sense of invincibility, denial, or embarrassment, and they may give an incomplete history, especially to a physician untrained to “read between the lines” of an adolescent history.
The disparity between maturation of the prefrontal cortex, which is responsible for executive control and rational decision-making, and the pleasure-seeking limbic system might underlie young AYAs’ orientation to the here and now as well as risk-taking behaviors such as prioritizing social events over treatment and poor adherence [83]. The actual rate of nonadherence in AYAs is unclear due to difficulties in defining and measuring it objectively. However, it appears that a supportive family relationship, promoting involvement in treatment decisions, and supporting participation in normal activities might enhance adherence, while a chaotic social environment is likely to have the opposite effect [84]. This underscores the importance of pre-emptive referrals of AYAs with cancer to social work and psychological services.
Adolescents are often poor self-advocates, preferring to “blend in” and not upset the status quo or question authority. Additionally, their parents may not advocate to the same degree as they would for younger children. This is exacerbated by the fact that most AYAs have poorly developed self-management skills and hence may struggle with medication management, arranging appointments, and other tasks which they relied on their parents to perform previously. These factors may result in disengagement with the health system and poor adherence. This highlights the importance of a designated cancer care coordinator to help advocate for the young person and promote the development of self-management skills as they navigate the complex healthcare system.
20.1.5 Summary
In sum, we are beginning to learn more about the components of quality care for AYAs and the barriers they face in achieving access to that care. Although there is some knowledge gained from studies comparing care at pediatric versus adult centers or from pediatric versus adult providers, this is limited, both because it does not tell us what components are responsible for any differences in outcome and because only the youngest of AYAs will be eligible for pediatric care. Recent efforts to develop AYA programs, as detailed below, will teach us much about the essential elements of “personal health services to achieve the best possible health outcomes” and will require us to shift our assessment of access.
20.2 Models of Care
It is recognized currently that AYAs with cancer are a unique group, with special characteristics and special health needs [85, 86]. With the recognition of their complex psychological and social needs [87], together with the awareness of their inadequate access to optimal cancer services and the lack of improvement in survival rates [88], it has became evident that AYA patients are particularly complicated to care for and that the traditional healthcare models address their needs poorly [89, 90].
The teenage and young adult period is an age of transition, and AYA patients are often the older patients in children’s services or the younger in adult services. In terms of healthcare delivery, it has been said that these patients inhabit a “no man’s land,” neither belonging to the pediatric nor to the adult worlds of oncology. On the other hand, they may also become subject to professional competition for patient “ownership,” paying the price of the shortcoming in communications and collaborations between these two worlds.
Nevertheless, it is evident that the last decade has been a key time for AYAs, and several dedicated projects have been developed in an attempt to address the unmet needs of this age group. Starting a specific project, however, remains a challenge: the enthusiasm of people has to be able to counterbalance the obstacles of ingrained cultures (or even strong opposition), physical space constraints, administrative and logistic issues, low prioritization, and costs [91].
20.2.1 What Makes Adolescents and Young Adults Different?
There is substantial evidence of specific clinical and psychosocial features, and also specific challenges in patient’s management, that mark out being an AYA with cancer. Patients in this age group have specific clinical features because of their cancer types, tumor, and host biology; because of the insufficient awareness that cancer may occur in this age group and the complex diagnostic pathways; and because of the specific psychological and social characteristics that care providers must address adequately (Table 20.3) [92–99]. On the other hand, there is growing evidence that these patient features combine unfavorably with the features of many current healthcare systems (Table 20.4) [91, 100–110].
Table 20.3
Clinical and psychosocial parameters specific to adolescents and young adult (AYA) cancer
Feature | Comment and open questions |
|---|---|
Epidemiology: this age has a unique spectrum of cancer types: AYA may have pediatric-type and adult-type tumors; then there are tumors with a peculiar incidence pick in this age [92] | Multiple parts of cancer research and care need to work together to improve outcomes for AYA. Detailed outcomes are poorly understood, because of international variations in cancer registration, and limited linkage of registration to treatment and outcomes |
Tumor biology and clinical behavior of various tumor types are not the same in AYA as in younger or older people [45] | Management not simply the application of a children’s or adult standard of care |
Most AYA tumors may have unexploited identifiable specific tractable biological targets. There is little detailed prospective biological data linked to treatment and outcome for AYA tumors | |
The biology of an AYA patient (not just their cancer) is distinct. AYA may have specific pharmacology and toxicity profiles that may be relevant in treatment protocols [94] | Treatment protocols tailored for children (or adults) may be not simply applied to AYA |
It remains to be clarified whether these differences in biology (in cancer and host) may explain the differences in outcome | |
There is insufficient awareness that cancer may occur in this age group, among teenagers, their families, and physicians [95] | Diagnostic pathways may be complex, with multiple consultations and consequent therapy only after an unacceptable delay. Which improvements in professional and public education will reduce time from symptoms to diagnosis? |
Unique complex psychological needs (physical changes, development of self-image, identity, relationships, and sexuality, seeking independence; communication challenges, compliance, and treatment adherence; peculiar behaviors of this age and risk-taking) (smoking, alcohol, substance use, sexual health) [87] | These normal needs may go unrecognized as what they are or even be resented by some professionals. Information and consent must be tailored to a patient’s age and level of maturity. What are the predictors of psychosocial adaptation after AYA cancer? |
Fertility preservation is a key issue for AYA [96] | Addressing this issue actively is an essential part of the overall management |
What is the balance of parental, sibling, and personal responsibility in adolescent decision-making for their future? | |
Distinct late sequelae (e.g., late cardiac toxicity, secondary tumors, psychosocial reintegration into life) [97] | Specific management and follow-up may be necessary. Which model should we follow for managing deferred risks in adolescents? Is lifelong follow-up the best option? |
Transitional programs (to take care for children as they become older, and move patients through to adult services when older still) [98] | AYA services need open collaborative cultures and funding mechanisms to support care. How do we hold vulnerable people’s implicit trust at the time of diagnosis while not hindering a transition of care to others in the future? |
Peculiar aspects in palliative care, death, and bereavement (adjustment to a short life expectancy in the AYA age is complex) [99] | Balance of pediatric and adult palliative care skills. Do AYA services need more specific approaches to end of life care? |
Table 20.4
Specific challenges in managing AYA cancer
Feature | Comments |
|---|---|
AYA patients can benefit from expert management including a specific integrated multidisciplinary team [91] | This should include access to psychologists, education and vocational mentors, nurses, social workers, activity coordinators to continue developmental social activities during stays in hospital |
Each professional needs training in the specific issues of AYA | Need of joint adult and pediatric credentialing organizations. Doctors trained in all of AYA care (regardless of their pediatric or adult oncology background) may provide better care |
AYA with cancer may seem only to accept a curative intent [100] | Management of AYA cancer needs to reflect their frequent ambition to seek a cure (even in situations where the chance to reach this is low). A prolongation of life by some years with improved control of symptoms that is frequently achieved in older adults receiving effective modern cancer treatment is less relevant to AYA |
The reasons of these differences probably combines factors linked to the biology of tumors, factors related to delivered treatment and factors due to the patients themselves (e.g., delay in diagnosis, adherence and specific pharmacology) | |
AYA may have limited access to cancer services which meet their needs [103] | Many patients are managed either as younger children or as older adults would be |
Policy changes at different rates in different nations may lead to improvements | |
Developing clinical research protocols is a fundamental part of cancer research. Entry in clinical trial has been suggested as a factor improving survival | |
Need to facilitate and prioritize trial entry | |
Clinical trial entry criteria disadvantage AYAs [104] | Trial entry criteria reflect the practices of the clinicians designing the trials, not the disease under study. The trial entry criteria may reflect the practices of the clinicians designing the trials, not the disease under study. Trials in cancers should have age-related entry criteria that offer recruitment to all AYA with comparable tumor biology |
AYA may fall between children’s and adult services, but may also be subject to professional competition for patient “ownership.” It appears neither a pediatric nor an adult clinical group can manage this group of patients without the active collaboration of the other group. Currently however cooperation between pediatric and adult oncologists could/should be improved | |
Young people strongly prefer to be cared for alongside their peers, rather than with younger children or older adults [108] | An adequate setting and environment for this care is required as choice for AYA to make. |
There is the necessity to consider also AYA specific needs for privacy from other peers, but also their need for social contact | |
Many young patients are eloquent advocates for the services they value [109] | It may be helpful to actively promote the patient voice at the center of services. |
A partnership with AYA and their groups, parents and their associations where involved, and charities, may be critically important to shaping a service meeting AYA needs, and involving people who are harder to reach | |
AYA specialization is a recent consideration [110] | For AYA, and the clinical culture, investment and networks have existed for a shorter time and are therefore less mature than those dedicated to children or adults. |
There is a need for infrastructure investment to establish these services |
One of the main challenges for young people with cancer is the possibility to continue to live as normal a life as possible and to achieve developmental tasks. This critical concept needs to be taken into account when developing age-specific models of care. In this group, people change their perception of the world and need to test their limits. A recent review described brilliantly the AYA’s world with the reference to the famous phrase of “sex, drugs, and rock ‘n’ roll” [111]. Care providers should be able to address the need of young people to live their experiences and rites of passage, that cannot be postponed due to the event of a cancer diagnosis. Care providers should be able to establish an open relationship with their patients to help them to revisit these aspects in the light of their cancer history. Young patients need to feel comfortable disclosing sensitive matters. Talking about “sex” – about sexual identity and sexual practices but also sexual dysfunction related to the disease or treatment – may be particularly complicated for a teenager in front of a doctor. A sensitive, nonjudgmental, and confidential relationship is essential also regarding “drugs” – alcohol and drug exploration and use. Clinicians must be aware of aspects such as the negative impact of alcohol and drug use (or abuse) in relation to the patient’s health, the interactions with medical drugs, and the associated psychosocial morbidity; but they should be able to address this issue in a timely and appropriate manner, avoiding a paternalistic approach and prohibition that, for example, may have a negative impact on treatment adherence. Alcohol consumption and recreational drug use may have a role for socialization and for feeling normal and being part of the crowd. An adequate balance should be considered appropriately to help young people in developing their sense of self and their feelings of connection to the world. The “rock ‘n’ roll” lifestyle distinguishes young people from children and from older adults. The crucial concept that care providers dealing with AYAs should have in their mind is that a patient is first a normal young person who happens to have a cancer: this concept should be at the foundation of the model of care. Promoting normalcy means adequate spaces and rules in the ward to facilitate the relationship with peers and prevent the isolation that long periods of hospitalization may imply: social zone and multifunctional spaces with technical equipment (TVs, computers, musical instruments, books, magazines, and DVDs appealing to the age group concerned), for example, as well as kitchen/dining zone or “chill-out” zone. It means accessible visiting times and easy access to the hospital. It means the availability of activities and events as take away evenings. But it means also the opportunity to respect the need of privacy and the possible wish to refuse the involvement in social activities.
A further critical aspect relates to what young people say they need. In designing and delivering services, it is essential to take the experiences and views of patients into account. It is important that the young people are given a “voice and a choice” in their care, as this helps to inform and underpin everything that is developed for them. Given the opportunity, young people will say what they want from services and those who provide them.
20.2.2 Current Healthcare Delivery Models: Medical Oncology Versus Pediatric Oncology
Pediatric oncologists and adult medical oncologists usually adopt different organization models, particularly as concerns interaction with the patient. With a rough generalization, it could be said that the pediatric model is family focused, while the adult model seems to be disease focused (Fig. 20.1).
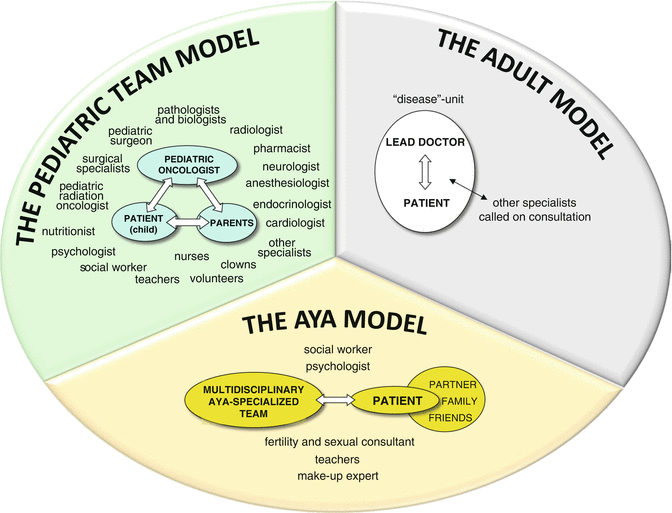

Fig. 20.1
Healthcare delivery models: the family–focused pediatric model, the disease–focused adult model, and a possible model for AYA
20.2.2.1 Pediatric Oncology Model
The pediatric model of care is based on a complex, sometimes dualistic, relationship between three leading actors, i.e., the child, the parents, and the healthcare professionals. The pediatric oncologist has to establish a deep relationship with both patient and family, but the interactions are on different levels: for instance, parents are fully informed about the child’s condition and prognosis and involved in the decision-making process, while the child himself/herself is often not [112]. As children get older, their contribution to decision-making may become more apparent, although decisions and discussions remain an intricate process in which parents’ views tend to predominate [91].
Another important characteristic in pediatric oncology units is that, rather than assembling consultants piecemeal, the young patients are managed routinely by an integrated multidisciplinary staff of pediatric oncologists, surgeons, radiotherapists, nutritionists, and subspecialists in infectious disease, neurology, and endocrinology, as well as nurses, teachers, psychologists, social workers, and others.
Pediatric oncology teams have been relatively well resourced, with a high staff/patient ratio and a greater amount of time given in support and interaction with patients and families [91]. Though pediatric cancers account for less than 1 % of the total cancer burden, society and the healthcare system have accepted a disproportionate allocation of resources to children and their families in the view of the differential societal burden of years-of-life lost, which is estimated to be 69.3 years for childhood cancers compared to 15–20 years for the most common adult malignancies [113].
Finally, in pediatric oncology, care is often given on or according to standardized protocols or clinical trials, with centralization in a limited number of referral centers. Historically, pediatric oncologists have put a lot of effort into promoting national, and ultimately international, multicenter collaborative networks: pediatric patients usually have a good chance of being treated within cooperative clinical trials, whereas the proportion of adult patients entering multicenter clinical trials is far lower. This is partly a function of resources but also reflects fundamental differences in trials strategy. For example, adult medical oncologists place a relatively greater emphasis on phase 1–2 trials and research on new treatments (and on randomized phase 3 trials tailored to the “big killers”), while pediatric oncology trials are usually in phase 3 (randomized or risk based) and the emphasis is often on limiting the burden of therapy to reduce toxicity and sequelae (without jeopardizing the results achieved in some diseases).
20.2.2.2 Adult Medical Oncology Model
Professionals engaged with adult patients tend to work within a more classical medical model of a lead doctor interacting directly with the patient, often within units dealing with specific types of tumor. This doctor–patient relationship is at the core of practice and is based on confidentiality and consent. Patient autonomy is central to the therapeutic relationship: the patient rather than the family is at the center of this particular care paradigm, and other family members or partners interact with the professional team largely through the consent of the patient.
Although a multidisciplinary approach has been implemented increasingly at adult units too, particularly at referral centers, this often refers to the involvement of the surgery and radiation disciplines within a clinic program dealing with a specific type of tumor, while it remains rare to encompass psychosocial, nutritional, or educational support routinely.
Though there is more variability of care models in adult oncology, the staff/patient ratio is generally low, and resources have been more stretched. This implies differences in time and resource allocation (i.e., different amounts of time spent on interacting with patients) as well as costs. It remains to be seen whether it might imply also differences in global quality of care and patient satisfaction.
Historically much of the focus of adult teams has been on older patients (and there is much logic in this as the number of older patients who develop cancer is large). In many cases, the emphasis has been often on treating with largely palliative intent while paying particular attention to unwanted acute side effects. The proportion of patients entered into multicenter clinical trials is much lower than in pediatric oncology [88].
20.2.3 The Ideal Model of Care for AYA
Since AYAs are neither children nor older adults, and yet share many characteristics of both, it is not surprising that neither of the classic pediatric or adult models of care is ideally suited to meet the needs of AYA patients. Certainly, it remains to be seen whether a single, ideal “new” model of delivery of care should exist for AYA patients and whether it could feasibly be implemented. Alternatively, there should be discussion about what adjustments should be made to one or both systems to better meet the needs of the AYA patient [114].
Ideally, an AYA-tailored model of care would have to be patient focused: the doctor needs to interact directly with the patient, with sufficient sensitivity to acknowledge each patient’s level of maturity and independence and unique needs. Interacting with a patient’s parents and/or other figures, such as a partner or friends, still has an extremely important role, given the wide range of independence encountered across the AYA stage of development. Moreover, sensitivity and flexibility are required to meet the needs of each individual, in view of the different levels of maturity and independence.
Due to the complexity of their care and the variety of their psychosocial issues, a multidisciplinary approach is necessary. In addition to the routine group of specialties, this may well include nurse educators, navigators, fertility experts, social workers (especially skilled in employment and insurance counseling), teachers, psychologists, sexual consultants, and even a cosmetics expert (Fig. 20.1).
Given that teenagers and young adults develop a wide range of cancers that encompass both pediatric and adult types of tumors, the multidisciplinary team should include the expertise of both pediatric and adult medical oncology. Providing both of these skills remains a challenge. On the one hand, the direct presence in the team of both pediatric oncologists and adult medical oncologists may be an apparently easy solution. On the other hand, the figure of a “new” tailored healthcare provider – an AYA oncologist – may be advisable. Barriers exist for both solutions [91].
Establishing a genuine collaboration and shared ownership between pediatric oncologists and medical oncologists remains a difficult goal. There are different backgrounds, different priorities, and different models of working: even when they deal with similar diseases, pediatric and adult oncologists often adopt different classification, staging, and grading systems, as well as different practices relating, for example, to data collection – and consequent difficulties when it comes to sharing data. Though both may have much to gain from cooperating with one another, competition often exists. Historical experience showed that AYA dedicated programs have developed in some cases as an adjunct to the local adult oncology facility, while in other cases they have developed predominantly as an adjunct to pediatric oncology services. It has been less common for units to be developed by joint collaboration between adult and pediatric oncology teams.
The ultimate solution to develop oncologists (and oncology centers) specifically dedicated to the care of AYA patients – to create a new discipline, i.e., AYA oncology, with its own training programs, clinical and translational research, and national and international organizations – still remains a challenge. For example, current training for either pediatric or medical oncologists does not provide the necessary skill, and a modification of current fellowships would be necessary.
However, more than regarding the specific training needed for AYA oncology, the key unresolved issue remains whether, in practice, the AYA oncologist ought to cross all disease boundaries, treating breast cancer, melanoma, rhabdomyosarcoma, and leukemias with equal competence. Given the complexity of modern oncology, this seems unlikely. As a matter of fact, a tension continues to exist between providing centralized care in a unit specializing in a particular malignant disease or in a unit specializing in the care and needs of young people. It might be thought that the best solution for young people would be to create an environment that combines these elements to ensure that teenagers and young adults benefit from both. The optimal model may be a unit designed specifically for young people and staffed by a skilled multiprofessional team expert in both the care of teenagers and young adults and their diseases and committed to working in new ways. In this way, the role of the AYA oncologist may be complementary to these of pediatric and medical oncologists. Patients would be cared for in a unique space (inpatient and outpatient) with specially trained providers, within a culture that recognizes the various needs of young patients as well as the necessity to improve clinical trial enrollment [91].
20.2.3.1 Key Themes in Developing an AYA Oncology Program
Various experiences of AYA oncology programs have been developed – and are in development – all over the world, with differences and similarities (discussed above in this chapter). The possible identification of an ideal model of care for AYA oncology is still a challenge, in particular because AYA units cannot be formed instantly or in isolation. In practice, developing a dedicated program should reflect not only an ideal but also acknowledge local issues and variations in medical culture and resources which have and will continue to generate an interesting heterogeneity of solutions.
A recent review has tried to underline the key elements that needed to be considered and developed according to local issues in starting an AYA-tailored program:
Cooperation between pediatric and adult medical oncologists – while it is true that many of the existing schemes have arisen in the pediatric oncology setting, several have succeeded when originating from the medical oncology side; real results can only be achieved if there is a genuine cooperation between, and leadership by, both pediatric oncologists and medical oncologists.
Definition of “who AYA are” – any local project should be able to define in advance its target in terms of the age cohort (from 15 to 24, 29, or 39 years old?).
Staffing – although appealing, it is unlikely that most programs will be able to create a new unit of AYA oncology with multiple specialties represented (each with an AYA focus and expertise), while it is possible that team members may be involved as “part-time” figures, with other responsibilities in a home department. In some cases, the decision has been that of focusing more on dedicated non-physician staff (nursing, psychology, social work, child life). The development of specialized teams should be based on the recognition that the staff needs special training
Availability of clinical trials for all the tumor types occurring in the AYA age group – the inadequate inclusion in clinical trials has been demonstrated as one of the factors responsible for the lack of recent improvement in survival rates for AYA patients as compared to other age groups and should be seen as a critical theme to address. Providing dedicated physical areas – as done in some programs – as well as adequate psychosocial support facilities should be considered insufficient if patients are not enabled to enter clinical trials.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree