Minimally invasive ablative therapy techniques are being used in research protocols to treat benign and malignant tumors of the breast in select patient populations. These techniques offer the advantages of an outpatient setting, decreased pain, and improved cosmesis. These therapies, including radiofrequency ablation, cryotherapy, interstitial laser therapy, high-intensity focused ultrasonography, and focused microwave thermotherapy, are reviewed in this article.
Over the past 5 decades, the treatment of breast cancer has evolved from highly invasive radical mastectomies to breast-conserving therapy that allows women to avoid severe disfigurement and related morbidity while providing equivalent survival benefit. More recently, the approach for nodal staging has also become less invasive with the adoption of sentinel lymph node biopsy as the current standard of care for patients with early-stage breast cancer.
Continuing with this trend toward minimally invasive therapy, various ablative techniques have been studied for the treatment of breast cancer. Both thermal and cold techniques have been evaluated as potential treatment modalities. Radiofrequency ablation, interstitial laser therapy, high-intensity focused ultrasonography, and focused microwave ablation result in tumor destruction from significant heating of the tissue. Cryoablation is the technique that causes tumor destruction through extreme cooling of the tissue. This article gives an overview of the technical aspects of each procedure, along with the advantages, disadvantages, and evidence of their efficacy.
Radiofrequency ablation
Technical Aspects of Radiofrequency Ablation
Radiofrequency ablation (RFA) has been used for the treatment of liver metastases, primary hepatocellular carcinoma, pulmonary neoplasia, and renal cell carcinoma. RFA is performed by placing electrode tips within the lesion, creating a high-frequency alternating current into the surrounding tissue. The ions in the tissue attempt to follow the changing direction, causing ion agitation and thus frictional heating of the tissue. This process results in destruction of the tissue through thermal coagulation and protein denaturation. Thermal injury begins at 40°C, with the exposure time necessary for cell death decreasing exponentially at 42.5°C. Temperatures generated by the electrode must be much greater than 50°C to achieve cell death, as most human tumors require temperatures greater than 45°C to 50°C. The multipronged RFA probe is most commonly placed under sonographic guidance and the procedure performed during real-time sonographic monitoring. Treatment is completed once the tumor has been heated to a specific temperature or once tissue impedance has reached a level at which no further heating can occur.
Treatment of Invasive Breast Cancer with Radiofrequency Ablation
RFA has been described for the treatment of invasive breast carcinoma. Jeffrey and colleagues reported a small series of 5 women with tumor sizes ranging from 4 to 7 cm measured by clinical examination or mammography at the time of the procedure, who were treated with RFA at a single site within the tumor under ultrasound guidance. Following RFA, 4 patients underwent modified radical mastectomy and 1 underwent breast-conserving surgery. Complete loss of cell viability, as determined by NADH-diaphorase staining, was observed in the tumors of 4 patients, while a single small area (<1 mm) of cell viability was identified in one patient’s tumor. Hematoxylin and eosin (H&E) staining revealed 2 patients with necrosis of all cells and 3 patients with normal-appearing cells interspersed between necrotic cells. Although only a small number of patients were included, the results of this study were encouraging to further evaluate RFA in a larger group of patients.
The first prospective clinical protocol to evaluate RFA for invasive breast cancer was reported by Fornage and colleagues at the University of Texas MD Anderson Cancer Center. Twenty patients with 21 tumors that were well visualized on sonography were included, and all patients had tumors measuring 2 cm or less in greatest diameter, with 1 cm between the tumor and skin and tumor and chest wall. The RFA procedure was performed percutaneously under ultrasound guidance in the operating room immediately before the scheduled surgical procedure. Eleven patients underwent partial mastectomy and 9 underwent total mastectomy. Sentinel lymph node mapping was performed prior to the RFA procedure. Ultrasound changes as a result of thermal injury were monitored at random intervals throughout the RFA procedure. Only a single RFA ablation session was needed in all but one tumor. No skin or chest wall complications were identified intraoperative or postoperatively. On histologic examination of the surgical specimen, all tumors were found to have cellular damage and thermal effect (as observed on H&E staining) as well as complete ablation (determined by NADH-diaphorase staining). The studies by Fornage and colleagues and others that evaluate RFA within a short time period before the excision of the primary tumor are all limited because cell death can be delayed for 48 hours following ablation. In addition, evaluation of complications is limited by excision of the lesion and overlying skin that may have been affected.
To assess the delayed effects of RFA, Manenti and colleagues performed RFA followed by surgical resection 1 month later. This study enrolled 34 postmenopausal women with biopsy-proven, unifocal, invasive ductal carcinoma 2 cm or less in size, visible on both ultrasonography and magnetic resonance imaging (MRI) that was at least 1 cm from the skin and chest wall on sonography. Sentinel lymph node biopsy was performed at the time of the RFA procedure. MRI was performed 1 week and 4 weeks following RFA to determine the accuracy of MRI in predicting the efficacy of RFA. Definitive surgical intervention with lumpectomy was performed at 4 weeks post-RFA in all patients. Seven patients with positive sentinel nodes underwent axillary lymph node dissection. Patients also received breast irradiation or adjuvant systemic therapy according to St Gallen oncologic criteria, and hormonal therapy was given in accordance with the biopsy hormonal receptor status. H&E staining of surgical specimens showed a spectrum of changes, from partial (6% [2/34] of patients) to complete changes (94% [32/34] of patients). In addition, NADH-diaphorase revealed no viable cells in 97% (33/34) of the tumors. One week after ablation, MRI showed no residual suspicious enhancement in 31 of 34 lesions, although residual enhancement was present in the remaining 3 lesions. The correlation between MRI findings and pathology of the specimens was not described except in one patient in whom NADH-diaphorase staining confirmed residual cancer at the site of enhancement on MRI. Cosmesis was rated as excellent in 82% (28/34) of patients, good in 15% (5/34), and poor in 3% (1/34). Suboptimal cosmetic outcomes were attributed to the formation of a mass as a result of thermocoagulation and hyperpigmentation from superficial skin burn.
Finally, studies have been performed in which the primary tumor was not excised following RFA, with the objective of assessing recurrence. Oura and colleagues evaluated 52 patients with small (2 cm) localized breast cancers, confirmed on mammography, sonography, and MRI, who underwent RFA. Sentinel lymph node dissection was performed before the RFA procedure. The RFA needle was inserted from the areola into the tumor under ultrasound guidance. Cytologic evaluation performed 3 to 4 weeks post-RFA (presumably by percutaneous biopsy) revealed degenerative cancer cells in 30 patients and no cancer cells and/or degenerative materials in 22 patients. MRI performed 1 to 3 months after RFA revealed no evidence of residual tumor in the ablation zone. Ultrasonography showed residual tumor in 30 of the 52 patients, although no vascular flow was seen in any of the cases. With a mean follow-up of 15 months (range, 6–30 months), no patients had developed in-breast recurrence, locoregional recurrence (LRR), or distant recurrence. Cosmesis was determined to be excellent or good in 43 and 6 patients (83% and 11%), and fair in 3 patients (6%). One patient did experience a skin burn, identified shortly after biopsy. Similarly, in a pilot study of 29 patients (30 breasts) by Yamamoto and colleagues, no hypervascularity of the tumor in the ablated zone was found in any patient on post-RFA MRI. However, H&E staining of post-RFA tumor specimens obtained by vacuum-assisted biopsy was performed in 3 of the 29 breasts, revealing remarkable degenerative changes. The remaining tumors were diagnosed as containing viable tumor tissue. NADH-diaphorase staining showed no viable tumor tissue in 24 of the 26 patients in whom it was performed. Adverse events occurred in 4 out of 29 patients in this study. Fibrotic tissue was present in the tumors of all patients, confirming the absence of viable malignant cells in the samples analyzed.
Most studies using RFA have focused on using the minimally invasive approach for the treatment of invasive breast carcinoma. However, a novel role of RFA as an adjunct to surgical excision (eRFA) has been explored in an attempt to decrease the rate of reexcision and improve locoregional recurrence. In a pilot study, RFA was performed on an ex vivo lumpectomy specimen in 19 patients. Proliferation cell nuclear antigen (PCNA) staining confirmed 100% nonviable tissue within the ablation zones. In a subsequent group of 41 patients with T1 and T2 tumors (mean tumor size 1.6 cm), standard lumpectomy was followed immediately by eRFA. On final pathology, 10 patients had close margins (8 with 1 mm, 1 with 2 mm margin, 1 with focally positive margin) and none of these patients underwent re-resection. Seventeen patients received post-eRFA breast irradiation (41%). Two patients developed recurrent cancer in the same breast. One patient developed a new invasive lobular carcinoma 1 year following excision and RFA of ductal carcinoma in situ (DCIS). The recurrent tumor was located 8 cm from the primary tumor site. The second patient developed a new grade II invasive carcinoma following treatment of a grade III primary tumor 2.3 years after treatment, and the recurrent lesion was located 5 cm from the primary tumor site. Both patients were successfully treated with salvage mastectomy.
Conclusion
Although initial studies in the use of RFA for the treatment of early-stage breast cancer appear hopeful, there are several issues that need consideration before the technique can be widely adopted. The availability of pre-RFA tissue to evaluate estrogen, progesterone, and HER 2/neu status is imperative because loss of hormonal and HER 2/neu expression can occur with RFA treatment. In addition, RFA may potentially alter the lymphatic drainage pattern of the breast tumor. Thus, the timing of sentinel lymph node biopsy in conjunction with ablative techniques is controversial. To date, there have been no prospective trials to evaluate the influence of the RFA procedure on sentinel lymph node biopsy. To accurately assess the efficacy of RFA for invasive breast cancer, the technique must be evaluated in a prospective fashion with concomitant evaluation of resected surgical specimens. Resection should be performed at least 48 hours after RFA to allow for delayed histologic changes, and NADH-diaphorase staining may also be helpful in assessing cell viability. Thus far, RFA with subsequent surgical excision has only been evaluated prospectively in a relatively small number of patients. Thus, further evaluation in a larger number of patients with longer follow-up is needed to determine its role in the treatment of invasive breast cancer.
Cryotherapy
Cryoablation is an ablative therapy that is receiving increasing attention and is being used with increased frequency, due to improvements and advances in imaging and cold technology. It has already been studied in tumors of the liver, prostate, and kidney. Cryoablation is another example of a minimally invasive approach under investigation to treat breast tumors, both benign and malignant.
Technical Aspects of Cryoablation
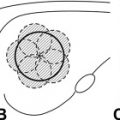
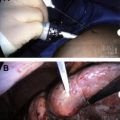
The technique of cryoablation uses cold technology to destroy a breast tumor in situ via localized freezing. After infiltration of local anesthetic, a small incision a few millimeters in size is created for percutaneous cryoprobe placement ( Fig. 1 ). The probe is inserted into the tumor via image guidance (usually ultrasound; however, computed tomography and MRI have also been reported) through a trocar ( Fig. 2 ). Nitrogen or argon gas (cryogen) flow is initiated into the probe tip via a computer modulated regulator. The gas undergoes a constant enthalpy expansion process, allowing a rapid local freezing reaction to occur. Then a second gas, such as helium, which warms when it expands, is released through the probe to arrest the freezing process and allow thawing to actively occur. The thawing process can also occur passively by discontinuing the flow of argon and allowing the probe to warm. This cycle is repeated and results in tissue necrosis. A complete treatment consists of at least 2 freeze-thaw cycles, with cycle length in minutes based on tumor diameter. Tissues exposed to at least 2 consecutive freeze-thaw cycles at temperatures of −40°C or lower will allow consistent and symmetric ablation to occur. An ice ball is created, which can be easily visualized on intraprocedural ultrasonography by visualizing the hyperechoic rim that forms between frozen and unfrozen tissue ( Fig. 3 ). The ice ball that forms creates a uniform ablation zone, providing an even field of distribution for the cryotherapy process, resulting in homogeneous tissue necrosis. However, there are reports that there is inconsistent cell death at the periphery of the ice ball resulting from differences in cellular exposure to the freezing temperatures of the cryoablation process, thus supporting the need for a double freeze-thaw cycle. The frozen tissue that has been created remains in situ and is reabsorbed by the body over time. Continuous ultrasound monitoring is used to monitor the extent of freezing based on the size of the forming ice ball. Saline can be injected between the tumor and overlying skin if there is a distance of less than 5 mm from the ice ball to the skin surface to prevent thermal injury to the skin from occurring ( Fig. 4 ). In this manner, superficial tumors may be treated. In patients who have larger tumors, multiple cryoprobes may be used to generate multiple isotherms within the target tissue to ensure complete tumor ablation. Of note, a core-needle biopsy needs to be performed prior to tumor ablation so that adequate tissue can be obtained both for a histopathologic diagnosis and for the determination of tumor receptor (ER, PR, Her2neu) status.
Tumor cells are destroyed in 2 ways, via direct cell injury and via indirect vasculature injury. In direct cell injury intracellular ice formation occurs, causing a shearing and rupture of membranes, which is an irreversible event. Extracellular ice formation occurs, creating a hypertonic environment and causing water to flow out of cells due to osmosis. This process, referred to as “solution effect injury,” causes cellular dehydration and damage. When thawing occurs, water flows back into the cell, increasing the intracellular volume and causing lysis to occur ( Fig. 5 ). Vascular injury occurs when the freezing process causes vasoconstriction. The vasoconstrictive process causes ischemia to occur, which results in damage to the microvascular endothelium. Platelet aggregation occurs, resulting in thrombosis and vascular stasis. This loss of circulation causes cellular anoxia and death as well as the production of free radicals.
Cryoablation as Therapy for Benign Breast Tumors
Because benign breast tumors are very common and can cause a significant amount of anxiety, fear, discomfort, need for ongoing surveillance, and impaired visualization on follow-up imaging due to mass effect from the tumor, definitive treatment in the form of cryoablation is a viable option for patients who do not desire surgery or are not candidates for general anesthesia or surgical resection. Several investigators have evaluated the efficacy of cryoablation for benign breast tumors. Kaufman and colleagues reported on a series of 57 patients with fibroadenomas of the breast treated in a prospective nonrandomized multicenter trial who received cryoablation as their primary therapy. The mean maximum tumor diameter was 2.1 cm. After histopathologic confirmation of a fibroadenoma by core biopsy, patients underwent cryotherapy using 2 freeze-thaw cycles, with freezing occurring at −40°C. Following cryoablation, patients were followed with clinical examination, ultrasonography, core-needle biopsy, and patient satisfaction surveys. Long-term follow-up results for 29 patients were available, with an average follow-up time of 2.6 years. The efficacy analysis showed a continuous decrease in tumor palpability and visibility on both physical examination and imaging, respectively, as tissue debris resorbed over time. Only 16% of lesions remained palpable at the time of last follow-up. A median volume reduction of 99% of the original tumor occurred per ultrasound imaging at the time of last follow-up. No major complications were reported; although 3 patients reported minor complications of tenderness (2) and cyclic pain (1). Ninety-seven percent of patients reported satisfaction with the results of the procedure, and 100% of physicians reported satisfaction with the procedure and the long-term results.
Littrup and colleagues reported on the subgroup of 29 patients who were treated at a single institution from the multicenter trial reported by Kaufman and colleagues These 29 patients had 42 fibroadenomas treated with cryotherapy. Thirty-seven of the 42 fibroadenomas had 12 months of follow-up. In 33 of these 37 patients, their fibroadenoma had either completely resolved or was much smaller on physical examination. None of these patients exhibited skin changes over the treated area or evidence of skin depression secondary to volume reduction at the treated site. Although there was initial hypopigmentation at the probe insertion site in 3 patients, this had resolved at 12-month follow-up; one patient developed a keloid. The investigators reported that both patient and physicians were “very satisfied” with the cosmetic outcomes. On Doppler ultrasound evaluation at follow-up, no significant flow was noted in the treated area, whereas there was normalized flow in the adjacent untreated tissue. By 12 months, continued shrinkage and resorption rendered 5 of the fibroadenomas no longer visible by ultrasound imaging. All of the patients were offered the opportunity to undergo surgical resection if they were unsatisfied with the results of the cryoablation; only 2 patients chose to have a formal resection.
Nurko and colleagues reported results from the prospective Fibroadenoma Cryoablation Treatment Registry, which includes patients with breast fibroadenomas treated with cryotherapy in a community setting. Four hundred and forty-four fibroadenomas with a median lesion size of 1.8 cm were treated at 55 different practice sites. After placement of the cryoprobe through the long axis of the fibroadenoma, 2 freeze-thaw cycles at probe temperatures of −160°C were used for treatment. Patients were followed at 12 months with clinical examination and ultrasound imaging. Thirty-five percent of the tumors that were initially palpable were palpable at 12 months for the 82 patients for whom this information was available. Ultrasound imaging showed the reabsorbing lesion in 29% of the 71 patients for whom this information was available. Patient satisfaction at 12 months was high (88%) in 84 patients. In contrast to surgical excision, cryoablation of fibroadenomas results in decreased morbidity from ductal damage and incisional pain, decreased patient distress, and improved cosmesis. As many physicians are reluctant to allow a lump to remain in situ in a woman who may not be motivated toward appropriate follow-up, cryoablation represents an effective minimally invasive alternative to surgical excision in these patients who have a biopsy-proven benign process.
Cryoablation of Invasive Breast Carcinoma
More recently, the role of cryoablation in the treatment of malignant breast tumors has been evaluated, primarily in early-stage cancers. Pfleiderer and colleagues treated 15 patients with 16 early-stage breast cancers (9–40 mm diameter) with cryoablation. Each patient underwent ultrasound-guided cryotherapy consisting of 2 freeze-thaw cycles under local anesthesia, followed by surgical resection 5 days after the cryoablation procedure. No major complications were reported. In patients whose tumor size was 1.5 cm or less, there was no residual invasive carcinoma in the pathologic specimens. In patients whose tumors were larger than 1.5 cm there was incomplete tumor destruction, with remnants of invasive carcinoma present in the histopathologic specimens. The investigators recommended the use of multiple cryoprobes to effectively treat larger lesions. In a follow-up study, Pfleiderer and colleagues prospectively evaluated 30 patients with biopsy-proven breast carcinoma (invasive and in situ) 15 mm or less in size who underwent cryoablation. Twenty-nine patients successfully underwent ultrasound-guided cryotherapy with 2 freeze-thaw cycles, with a median minimum temperature of −146°C. No major complications were reported. All patients underwent surgical resection within 6 weeks. No invasive tumor cells were identified in any of the patients, whereas DCIS was identified outside of the ablation zone in 5 patients, raising the question of the accuracy of pretreatment imaging in defining the extent of disease.
Sabel and colleagues performed a multi-institutional pilot study to assess safety and feasibility of cryotherapy in the treatment of early breast cancer. Twenty-nine patients with T1 tumors were enrolled in the study; 27 patients successfully underwent cryoablation with a double freeze-thaw cycle with a probe temperature of −160°C. Most patients (25/27) had the procedure performed under local anesthesia. All patients then had a formal oncologic surgical resection 7 to 30 days after the cryotherapy. Twenty-five of these 27 patients underwent a sentinel lymph node biopsy at the time of the surgical resection, with 4 patients having carcinoma identified in the sentinel node. There were no major complications. Minor complications that were reported include tenderness, edema, and ecchymosis. Cryotherapy successfully ablated 100% of carcinoma cells in tumors that were less than 1.0 cm in diameter. In patients who had tumors that were between 1.0 and 1.5 cm, cryoablation achieved 100% success only if the tumor did not contain a significant amount of DCIS. In patients who had DCIS present without calcifications, cryotherapy was not reliable. The investigators showed that cryoablation was safe and well tolerated in an office-based setting, with reliable results of complete eradication of all carcinoma cells in patients with ductal or medullary tumors less than 1.5 cm in size who did not have extensive intraductal component (EIC). Thus, the success of the procedure was based on the size of the cancer and the histology of the tumor. Sabel and colleagues also showed that sentinel lymph node biopsy could be performed after cryotherapy, although the false-negative rate was not specifically determined in this study. As breast cancers are detected at much earlier stages nowadays because of the increased screening and improved imaging techniques, having an alternative to surgical resection that produces less pain, improved cosmesis, and decreased costs is advantageous.
The studies by Pfleiderer and colleagues and Sabel and colleagues show that cryoablation can be used for definitive destruction of breast carcinoma in select patients. Tumor size and histology are important criteria in defining the appropriate patient population. Tumors must be unifocal and visible on preoperative imaging. Sentinel lymph node biopsy is feasible after cryoablation has been performed, although the issue of accuracy in this setting still needs to be evaluated.
Additional Roles for Cryoablation in the Treatment of Breast Cancer
Cryoablation can also be used for tumor localization prior to lumpectomy for nonpalpable lesions, similar to the needle-wire localization technique. To achieve this localization, a cryoprobe is used to enclose a nonpalpable or barely palpable tumor (with surrounding tissue margin) in a rim of ice under ultrasound guidance immediately before a formal breast-conserving surgical resection, creating a template for the resective procedure. This technique eliminates the need for an additional procedure outside the operating room, as is the case with needle-wire localization, and also minimizes the possibility of displacement of the localization device during patient transfer. In addition, the frozen tissue is more firm and easier to manipulate during surgical resection. Also, this procedure may potentially decrease local recurrence, likely by encapsulating the tumor and thus preventing the spillage of tumor cells during surgical resection. Of note, a core-needle biopsy would also need to be performed before surgical excision via the cryo-assisted lumpectomy technique, to ensure an accurate histopathologic diagnosis and receptor status identification because of the occurrence of freezing artifact after cryotherapy.
In addition, cryotherapy can be used for local-regional palliation of locally advanced breast cancer and Stage 4 disease. Tanaka treated 49 patients who had locally advanced, metastatic, or recurrent breast cancer with cryotherapy, which resulted in an improvement of symptoms. Specifically, the patients had less pain, control of hemorrhage, and tumor volume reduction, with a 5-year survival rate of 44%. Suzuki reviewed 8 patients who had locally advanced breast cancer with distant metastasis in whom the primary tumor was treated with cryotherapy. Cryoablation reportedly palliated symptoms of the primary breast tumor and palliated distant disease symptoms consisting of dyspnea due to a pleural effusion in one patient and lower extremity paralysis and incontinence due to vertebral metastases in another patient.
Several preclinical studies suggest that cryoablation may decrease local recurrence and distant metastases. In a murine model of breast cancer, cryoablation caused a tumor-specific immunologic response with a release of cytokines interleukin-12 and interferon-γ, and increased natural killer cell activity. This immune response may be able to prevent local recurrence and distant metastases. Sabel and colleagues have also recently reported a decrease in pulmonary metastases and an increase in survival in a murine model after cryoablation with a high freeze rate. Thus, a theoretical systemic antitumor response is created. In addition, cryoablated tissue has a surrounding hypervascular rim, which may have implications for delivery of chemotherapy or increased sensitization to radiation therapy.
Advantages of cryoablation include patient comfort, as the ice ball that is produced has an analgesic effect, minimizing the patient’s intraprocedural and postprocedural discomfort. There is improved cosmesis when compared with lumpectomy (without tissue rearrangement), as there is no volume reduction, retraction, or concavity within the breast tissue nor is there a large surgical scar ( Fig. 6 ). Also, the shape of the breast is maintained because the collagenous framework is preserved during the cryotherapy process. There is also increased convenience when compared with surgical procedures, as cryoablation can be performed in an outpatient setting without the use of general anesthesia, also decreasing overall costs. The procedure is well tolerated, and patients report a high degree of satisfaction with the treatment and the cosmetic results.
Disadvantages of cryoablative therapy center on the fact that certain staging information may be lost, due to a complete pathologic specimen not being produced; specifically, tumor size and margin status. Accurately defining tumor margins in relationship to the treatment field during and after the procedure is important. If this information is lacking, recommendations for adjuvant therapies may be altered or unclear. There is concern that the accuracy and ability to perform sentinel lymph node biopsy may be lost because of disrupted lymphatics if a sentinel lymph node biopsy is not performed before cryoablation, due to disruption of lymphatic channels that may occur with cryotherapy. However, Sabel and colleagues have reported that it is feasible to perform sentinel lymph node biopsy after a cryoablation. Also, it is also important to perform a core biopsy before cryoablation, ensuring enough tissue is obtained for an accurate diagnosis and tumor receptor status. Another disadvantage of cryoablation is that the ice ball that is created can remain for approximately 1 month after the procedure before it is resorbed by the body, interfering with physical examination or obscuring postprocedure imaging. The larger the tumor and subsequently formed ice ball, the longer it takes for resorption to occur. Also, current imaging modalities are limited in their ability to detect in situ carcinoma. Thus, if preablation imaging does not accurately define the extent of disease, cryotherapy may not destroy all in situ and invasive cancer.
Complications of the procedure include ecchymosis and hematoma formation. Thermal injury to the overlying skin in the form of ulceration or necrosis may also occur. As previously mentioned, this skin injury can be minimized with the injection of saline into the tissue between the tumor and the overlying skin. Minor complications include pain and tenderness at the site of the procedure. Postprocedural edema has also been reported.
Postprocedural monitoring can be performed with a variety of imaging modalities, including mammography, ultrasonography, or MRI. These imaging modalities allow visualization of the ice ball that has been created as a result of the ablative procedure. Ultrasound imaging shows the ablated tissue becoming more hypoechoic over time, as it involutes, resorbs, and reorganizes. Following treatment, Littrup and colleagues reported that color Doppler evaluation showed no significant flow in the cryoablation zone. Tissue sampling can also be performed to ensure that no cancer cells remain within a specimen, if the procedure is being performed in patients with breast carcinoma.
The American College of Surgeons Oncology Group is currently enrolling patients in a prospective, multi-institutional, phase 2 trial, ACOSOG-Z1072, cosponsored by the National Cancer Institute, that is investigating the rate of complete tumor destruction in patients with T1 unifocal invasive ductal carcinoma treated with cryotherapy. Secondary end points include the evaluation of the negative predictive value of MRI to detect residual disease, adverse events assessment, pain assessment, and evaluation of technical variables affecting the cryoablation procedure. Patients must undergo a core-needle biopsy for diagnosis and to determine receptor status prior to cryoablation. Cryoablation of the breast cancer will be followed by surgical resection and lymph node evaluation within 28 days ( Fig. 7 ). Imaging follow-up will occur with MRI. To be eligible for this study, patients may not have received neoadjuvant chemotherapy.
In conclusion, cryoablation may represent an alternative to surgical resection for breast tumors. It is an office-based procedure that causes minimal patient discomfort with improved cosmetic outcomes, and has potentially reduced costs. Cryotherapy has been shown to be a safe and feasible treatment for breast fibroadenomas. Current ongoing prospective multicenter trials, such as ACOSOG Z1072, will evaluate its efficacy in the treatment of breast carcinoma and determine whether a secondary immunologic response occurs with cryoablation that may alter the rate of local-regional recurrence and distant metastases. Long-term results from prospective trials will be necessary before cryoablation can replace breast conservation therapy in selected patients with early breast cancer.
Cryotherapy
Cryoablation is an ablative therapy that is receiving increasing attention and is being used with increased frequency, due to improvements and advances in imaging and cold technology. It has already been studied in tumors of the liver, prostate, and kidney. Cryoablation is another example of a minimally invasive approach under investigation to treat breast tumors, both benign and malignant.
Technical Aspects of Cryoablation
The technique of cryoablation uses cold technology to destroy a breast tumor in situ via localized freezing. After infiltration of local anesthetic, a small incision a few millimeters in size is created for percutaneous cryoprobe placement ( Fig. 1 ). The probe is inserted into the tumor via image guidance (usually ultrasound; however, computed tomography and MRI have also been reported) through a trocar ( Fig. 2 ). Nitrogen or argon gas (cryogen) flow is initiated into the probe tip via a computer modulated regulator. The gas undergoes a constant enthalpy expansion process, allowing a rapid local freezing reaction to occur. Then a second gas, such as helium, which warms when it expands, is released through the probe to arrest the freezing process and allow thawing to actively occur. The thawing process can also occur passively by discontinuing the flow of argon and allowing the probe to warm. This cycle is repeated and results in tissue necrosis. A complete treatment consists of at least 2 freeze-thaw cycles, with cycle length in minutes based on tumor diameter. Tissues exposed to at least 2 consecutive freeze-thaw cycles at temperatures of −40°C or lower will allow consistent and symmetric ablation to occur. An ice ball is created, which can be easily visualized on intraprocedural ultrasonography by visualizing the hyperechoic rim that forms between frozen and unfrozen tissue ( Fig. 3 ). The ice ball that forms creates a uniform ablation zone, providing an even field of distribution for the cryotherapy process, resulting in homogeneous tissue necrosis. However, there are reports that there is inconsistent cell death at the periphery of the ice ball resulting from differences in cellular exposure to the freezing temperatures of the cryoablation process, thus supporting the need for a double freeze-thaw cycle. The frozen tissue that has been created remains in situ and is reabsorbed by the body over time. Continuous ultrasound monitoring is used to monitor the extent of freezing based on the size of the forming ice ball. Saline can be injected between the tumor and overlying skin if there is a distance of less than 5 mm from the ice ball to the skin surface to prevent thermal injury to the skin from occurring ( Fig. 4 ). In this manner, superficial tumors may be treated. In patients who have larger tumors, multiple cryoprobes may be used to generate multiple isotherms within the target tissue to ensure complete tumor ablation. Of note, a core-needle biopsy needs to be performed prior to tumor ablation so that adequate tissue can be obtained both for a histopathologic diagnosis and for the determination of tumor receptor (ER, PR, Her2neu) status.