Ablative therapies remain a useful adjunct in the multidisciplinary treatment of patients with colorectal liver metastases not amenable to hepatic resection. This review summarizes the rationale, underlying mechanisms, techniques, complications, and outcomes of current and emerging ablative modalities.
Colorectal adenocarcinoma remains the third most common cause of cancer-related death in the United States, with an estimated 146,000 new cases and 50,000 deaths annually. Survival is stage dependent, and the presence of liver metastases is a primary determinant in patient survival. Approximately 25% of new patients present with synchronous colorectal liver metastases (CLMs), whereas up to one half develop CLMs over the course of their disease.
The past two decades have seen significant advances in the treatment of metastatic colorectal cancer. Improvements in systemic chemotherapy have allowed for both prolonged survival and the opportunity for more patients to benefit from hepatic resection of CLM—currently, the only potentially curable treatment option. Unfortunately, because of unfavorable tumor location, disease extent, or insufficient hepatic reserve, approximately 70% of patients with CLM are not candidates for hepatic resection. This has led investigators to seek alternative methods of treating those patients who are not candidates for hepatectomy. In this regard, multiple liver-directed therapies have been developed that offer an opportunity for local therapy for those patients for whom hepatic resection is not feasible.
One of the most widely accepted nonresection techniques for addressing hepatic tumors is local tumor ablation. The rationale underlying the use of local ablative therapies for metastatic colorectal cancer rests largely on the observed survival benefit after resection of CLM and three theoretic concepts :
- 1.
Tumor consolidation after highly effective systemic therapy (ie, successful eradication of micrometastases with residual, stable macrometastatic burden after systemic therapy). This theory holds that elimination of this consolidated tumor may yield a survival benefit.
- 2.
Oligometastases, that is, identification of a subgroup of patients with metastatic disease that is intermediate between completely absent and widely disseminated, which may benefit from complete ablation.
- 3.
The Norton-Simon hypothesis that effectiveness of chemotherapeutics is proportional to tumor growth rate, with the hope that tumor debulking results in a smaller-volume (and faster-growing) metastatic population of cells that are more chemosensitive.
Nonresectional liver-directed therapies can be broadly categorized as catheter based (eg, transarterial chemoembolization, drug-eluting bead therapy, and radioembolization) or intraparenchymal ablative techniques. This review focuses on the two most widely used intraparenchymal ablative modalities for CLM by surgeons—radiofrequency ablation (RFA) and microwave ablation (MWA). A discussion of cryoablation and of emerging ablative technologies (laser interstitial thermal therapy, irreversible electroporation, and high-intensity focused ultrasound [HIFU]) is also included.
Cryoablation
Cryoablation was described early in the evolution of liver ablative technologies and involves placement of a cryoprobe into liver metastases by open surgical, laparoscopic, or percutaneous approaches. The cryoprobe tip is then rapidly cooled using liquefied gases and, over sequential freeze-thaw cycles, forms an ice ball encompassing the CLM and a rim of normal liver. The progression of the ice ball can be easily monitored by ultrasound. Tissue destruction follows via multiple mechanisms, including membrane rupture, dehydration, protein denaturation, vascular stasis, and electrolyte disturbances.
Hepatic cryoablation has some significant limitations. Heat sink effects may limit effective treatment of CLMs in close proximity to intrahepatic vascular structures, which may not allow for sufficiently low treatment temperatures. This raises the potential risk of incomplete ablation that might result in a higher risk of local recurrence. In addition, little intrinsic hemostasis is achieved by cryoablation, and hemorrhage has been reported as a result of cracking of the ice ball formation. A potentially lethal cytokine-mediated systemic inflammatory response, termed cryoshock , has been described after cryoablation, with a frequency of 1%. Perioperative mortality after cryoablation is estimated at 1.5%. These complications as well as reports of inferior results compared with other ablative technologies have limited the use of cryoablation for CLMs at most centers.
Hyperthermic ablative technologies
The use of hyperthermia to treat tumors dates to ancient times, with the use of cautery to treat superficial tumors. Modern hyperthermic ablative technologies rely on exposure of tumors to supranormal temperatures to ablate intrahepatic tumors. In contrast to cryotherapy, where tumors are more resistant to freezing than normal cells, malignant cells are more sensitive to hyperthermic damage than normal cells. Tumors lack the ability to dissipate heat by augmenting blood flow that is found in healthy tissues.
For most tumors, exposure to temperatures above 42°C results in low-level thermal injury, with increases in injury noted with increased exposure time and temperature. At progressively higher temperatures, there is an exponential and inverse relationship between treatment temperature and the exposure time needed to achieve cell death—that is, higher treatment temperatures require less exposure time for successful ablation. Temperatures above 60°C lead to reliable cell death through complex interactions, involving apoptosis, microvascular damage, ischemia-reperfusion injury, Kupffer cell activation, altered cytokine expression, alterations in the immune response, RNA and DNA destruction, dissolution of lipid bilayers, and protein denaturation. Thermal coagulation begins at approximately 70°C, and tissue desiccation occurs at approximately 100°C.
Radiofrequency Ablation of CLM
RFA achieves local hyperthermia using high-frequency alternating electric current in the radiofrequency range (100–500 kHz). Local hyperthermia results from ionic vibration and frictional heating of surrounding tissues. RFA gained popularity in the 1990s after reports by McGahan and colleagues and Rossi and colleagues and serves as the prototypical ablation platform for most clinicians today.
Radiofrequency current is applied through an electrode that is deployed within the tumor, using ultrasound, CT, or MRI guidance. RFA probes were initially developed as single-needle electrodes but have evolved to multiprobe and internally cooled arrays with attendant increases in treatment volumes and more reliable geometric ablation zones. Several devices are marketed worldwide, and none has emerged as superior with regard to ablation size, reproducibility, or local tumor control.
Percutaneous, laparoscopic, and open surgical approaches have been successfully used for RFA treatment, each with inherent advantages and disadvantages. The choice of approach should be individualized based on tumor anatomy, extent of disease, and patient comorbidities. An open surgical approach to RFA is preferable for patients with large tumors, multiple tumors, and tumors near large blood vessels that may otherwise be inadequately ablated because of heat sink effects. Hepatic inflow occlusion (Pringle maneuver) diminishes the heat sink effect from large intrahepatic vessels and is easier with open surgery compared with laparoscopic surgery. An open approach also allows for extremely accurate intraoperative ultrasound, which facilitates ablation of large tumors near blood vessels or in difficult anatomic regions of the liver. Peripherally situated tumors may be safely ablated by packing adjacent organs away from the liver, thus providing a layer of protection difficult to achieve with other approaches. Thoracic transdiaphragmatic approaches to tumors near the dome of the liver have also been described, and this technique may be useful for patients in whom multiple prior surgical procedures have resulted in prohibitive perihepatic scar tissue.
Laparoscopic RFA is an option for patients with tumors for which percutaneous RFA is not feasible or safe, such as peripherally situated tumors near adjacent organs, such as the stomach or colon. Patients may benefit from the decreased incisional morbidities and faster recovery times afforded by a laparoscopic approach. Substantial surgical judgment must be exercised, however, in selecting patients for whom the laparoscopic approach is appropriate, and oncologic principles should not be compromised intraoperatively. Modern endoscopic optics and laparoscopic ultrasound probes permit excellent visualization, making this approach increasingly attractive.
Percutaneous RFA is well suited for patients with CLM who are not good candidates for more invasive procedures due to comorbid conditions. In general, percutaneous RFA requires tumor sizes and locations that can be accessed without damaging adjacent organs or vascular structures. Although CT-guided RFA is relatively easy for small tumors in the lower segments of the liver, tumors along the edge of the liver can pose exposure challenges. Artificially induced ascites or pleural effusions by injection of dextrose solution has been reported as a means of separating vulnerable nearby organs, thus potentially expanding this indication. Cirrhotic patients and patients with limited intrahepatic recurrences after hepatectomy are examples of patients who may be best served by a percutaneous approach.
Regardless of the device or approach used, attention to RFA probe placement is critical to successful tumor ablation. Careful assessment of preablation imaging studies (CT or MRI) and thorough ultrasound examination of both the tumor and surrounding hepatic anatomy during treatment assist with successful probe placement. Ultrasonography can monitor the progression of the ablation during RFA. Gas bubbles generated by an ablation may interfere with accurate ultrasonography of tissues deep to the electrode, which can hinder repositioning of the electrode for overlapping ablation zones when treating larger tumors. Initial ablation of the deepest portions of the tumor, followed by serial redeployment as the electrode is withdrawn may mitigate this effect. Imaging-related difficulties may be more pronounced with the percutaneous approach, because transcutaneous ultrasound may not be as accurate as intraoperative ultrasonography; however, an immediate post-RFA CT can be readily performed to assist with the assessment of the ablation zone.
Complication rates associated with RFA are low, ranging from 2.4% to 27%, depending on the threshold for defining complications. By compiling data from more than 1300 patients from 18 different studies, Scaife and Curley reported an overall mortality rate of 0.5%, a major complication rate of 2%, and a minor complication rate of 6% after hepatic RFA. The risk of hepatic failure is low after RFA, even in patients with abnormal hepatic parenchyma. For this reason, RFA seems particularly attractive for cirrhotic patients. Monopolar RFA requires careful placement of grounding pads before ablation, because inadequate electrical grounding has been implicated in full-thickness skin burns. Other potential complications after RFA include wound infections, intra-abdominal abscess, renal failure, hepatic abscess, biliary tract injury, pleural effusion, fever, pain, and minor hemorrhage. A postablation syndrome has also been described and is characterized by low-grade fever, malaise, chills, myalgia, delayed pain, and nausea and vomiting. This syndrome is usually self-limited and resolves within 10 days but must be differentiated from more serious postoperative complications. Both RFA and MWA can be safely performed in patients with implanted cardiac devices but require coordination with cardiologists and perioperative device interrogation.
Because there are no published randomized controlled trials comparing RFA and resection, the effectiveness of RFA in CLMs is largely based on several single-arm, retrospective or prospective studies. These studies have inherent flaws (eg, selection bias, differing endpoints, and varying definitions of eligibility for RFA or resection); however, the available data point to RFA as an effective treatment modality in improving survival in CLMs ( Box 1 ). In an American Society of Clinical Oncology clinical evidence review, Wong and colleagues reported wide variability in reported 5-year survival rates (14%–55%) and local recurrence rates (3.6%–60%) after RFA, which are indicative of variability in selection criteria, treatment experience/technique, and endpoints across multiple institutions.
Advantages of RFA
Lower complication rates
Faster recovery
Safer in patients with marginal liver reserve
Ability to treat percutaneously
Disadvantages of RFA
Lack of long-term outcomes data
Higher local recurrence rates
Lower overall survival rates
Limitations in treating large or multiple lesions
Despite the variability in study designs, the preponderance of evidence supports the superiority of resection over RFA and a benefit of RFA over systemic chemotherapy alone. Abdalla and colleagues compared 368 patients who underwent potentially curative procedures (resection only, resection with RFA, and RFA only) with 70 patients with liver-only disease who received only regional or systemic chemotherapy. This series used as a control group patients with unresectable disease confirmed at laparotomy rather than historical controls. The investigators noted significant differences in both overall recurrence rates (52% [resection only] vs 64% [resection plus RFA] vs 84% [RFA only]) and 4-year survival rates (65%, 36%, and 22% for resection, resection plus RFA, and RFA only, respectively).
Abdalla and colleagues reported a survival advantage for patients undergoing either resection with RFA or RFA alone compared with chemotherapy only ( P = .0017). Also, in a series by Berber and colleagues, 135 patients who were not candidates for resection treated with laparoscopic RFA, a median survival of 28.9 was longer than the historical survival of 11 to 14 months with chemotherapy alone. These data suggest that RFA, although not superior to resection, does expand the armamentarium of surgeons, allowing for treatment options beyond chemotherapy alone for patients not amenable to resection, with the potential for improved survival.
Tumor number has been shown to affect both survival and recurrence rates—patients with solitary CLM have a better outcome than those with multiple CLMs. Tumor size has been shown an important factor affecting the rate of local recurrence after RFA, with multiple groups associating CLM tumor diameter greater than or equal to 4 cm with increased rates of local recurrence. Whether or not this is a function of unfavorable tumor biology or a limitation of current ablative technologies is a matter of ongoing debate. The relationship between increasing tumor size (or number) and increasing risk of local recurrence highlights the need for early detection and intervention for low-volume CLM.
Choice of treatment approach (open, laparoscopic, or percutaneous) may also influence local recurrence risk. Eisele and colleagues and Kuvshinoff and Ota reported lower rates of local recurrence for open and laparoscopic RFA versus a percutaneous approach. Improvements in hepatic exposure and the increased sensitivity of intraoperative ultrasound allowed by an open or laparoscopic RFA approach likely contribute to the lower local recurrence rates after operative compared with percutaneous RFA. Additionally, open and laparoscopic approaches allow for visual inspection of the liver surface for occult lesions and of the peritoneal cavity for extrahepatic disease. The apparent superiority of operative RFA is most likely due to better visualization, better control of surrounding structures, and more sensitive inspection of the peritoneal cavity.
Microwave Ablation of CLM
MWA, like RFA, is a hyperthermic ablative modality used in the treatment of CLMs. MWA uses microwave frequencies (≥900 MHz) to stimulate water molecules in target tissues, with resultant heat generation and thermal ablation. Although RFA uses ionic agitation to produce heat, MWA induces rotation of water molecules with resultant rapid increases in temperature. MWA was first developed as a hemostatic adjunct to parenchymal transaction during hepatectomy. MWA gained in popularity (largely in the Eastern hemisphere) for the treatment of hepatocellular carcinoma and later for liver metastases. Recent approval for MWA devices has led to increased use within the United States.
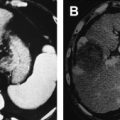
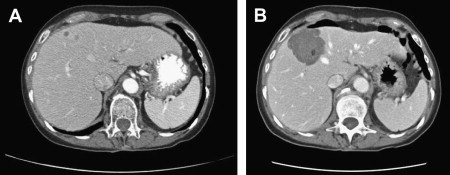
In clinical practice, MWA is similar to RFA in many respects, namely in selection of candidates, the device safety profile, and the approach to probe placement. Advantages of MWA include speed (median ablation times of 10 minutes), the ability to simultaneously ablate with multiple antennae, and lack of grounding pad complications. Other advantages have been suggested, with regard to larger active (as opposed to conductive) heating zones and in avoiding the limitations of increased impedance around RFA probes. Fig. 1 shows pre- and post-MWA CT images for two CLMs treated with simultaneous application of multiple MWA antennae.
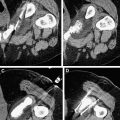
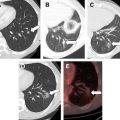
Probes for MWA include single and multiple antenna arrays as well as loop antennae for expanded ablation zones. As with RFA, MWA can be performed through percutaneous, laparoscopic ( Figs. 2 and 3 ), or open surgical approaches. The reasons for choosing one approach over another parallel the rationale for RFA (discussed previously).
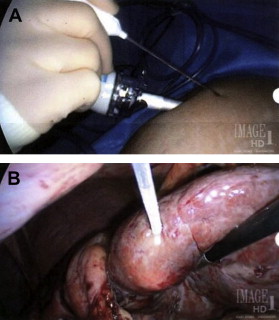
Although data are limited, survival and local recurrence rates after MWA seem comparable to those associated with RFA. The authors’ group has reported on 50 patients with unresectable CLMs treated by MWA. At a median follow-up of 3 years, recurrences at the ablation site were noted in 6% of patients, with a median disease-free survival of 12 months and a median overall survival of 36 months. As with RFA, MWA has also been employed in combination with hepatic resection for patients with CLM not amenable to one-stage resection. Tanaka and colleagues reviewed 53 patients with greater than or equal to 5 bilobar CLMs who underwent either resection or resection plus MWA. At a median follow-up of 21 months, there was no significant difference between the two groups with respect to overall, disease-free, or hepatic recurrence-free survival. The 3-year overall survival was similar for patients who required combined resection/ablation and those who underwent hepatectomy alone.
Laser-Induced Thermotherapy
Laser-induced thermotherapy (LITT) is another thermal ablative technology that uses low-intensity lasers (diode or Nd:YAG) to emit photons. These photons are then absorbed by natural molecular chromophores present in all human cells and converted into heat, resulting in cell death. LITT has similar indications as RFA, namely unresectable primary and secondary liver tumors and, like RFA, can be performed by percutaneous, laparoscopic, or open surgical approaches. As with other thermal ablative techniques, LITT ablation volumes can be increased by inflow occlusion, serial ablation, or placing multiple laser fibers into the target tissue, resulting in overlapping zones of ablation.
An advantage of LITT is its compatibility with magnetic resonance guidance. Unlike RFA or MWA, LITT does not interfere with MRI. Ultrasound or CT may be used for tumor targeting and placement of the laser fibers, followed by MRI of ablation progression. Real-time temperature monitoring is another potential advantage of LITT in conjunction with magnetic resonance guidance, because it allows clinicians to assure temperatures are high enough for successful ablation and low enough in surrounding tissue to minimize collateral damage. Currently, however, the limited availability of LITT, MRI guidance, and thermal mapping has precluded widespread use of LITT.
Few large-scale studies of LITT in the treatment of CLMs have been published. Vogl and colleagues, in a review of 603 patients with CLM, reported a median survival of 3.5 years and 5-year survival of 37% after diagnosis of metastases. Pech and colleagues reported on 117 colorectal metastases in 66 patients treated by MRI-guided LITT. At a short median follow-up of 8.7 months, a median progression-free survival of 6.1 months and a median overall survival of 23 months were d. An analysis of the complications associated with the LITT in 899 patients with 2520 lesions (primary and metastatic) concluded that the procedure had an acceptably low morbidity, comparable to other thermal ablative modalities.
Currently, LITT is available in only a few centers worldwide. Further multi-institutional studies are warranted to refine the feasibility, safety, durability, and efficacy of LITT in the treatment of CLMs.
High-Intensity Focused Ultrasound
HIFU is an emerging, noninvasive, thermal ablative technology that focuses acoustic energy within solid organs, resulting in temperature elevation and coagulative necrosis. Mechanical effects (inertial cavitation) are also observed in HIFU and contribute to cell death. HIFU transducers deliver intensities and compression/rarefaction pressures that are much higher than those seen in diagnostic ultrasound transducers.
HIFU is most successful in situations with good acoustic coupling between the ultrasound probe and the tumor (eg, prostate ablation or open surgical applications in the kidney). For hepatic HIFU, coupling between the skin and intervening tissues is suboptimal and has resulted in skin burns as well as rib necrosis. The ablation zone after a single HIFU ablation is typically cigar shaped and measures 1 to 3 mm × 8 to 15 mm. Treatment of larger volumes requires precise painting of the target lesion and can lead to difficulties in treating larger tumors in mobile organs, such as the liver. As with all thermal ablative modalities, the potential for incomplete ablation secondary to heat sink effect exists. Potential advantages of HIFU are largely related to its completely noninvasive approach. Continued refinements in techniques and technologies as well as prospective evaluations of efficacy in CLM may show it a viable future treatment option in the future.
Hyperthermic ablative technologies
The use of hyperthermia to treat tumors dates to ancient times, with the use of cautery to treat superficial tumors. Modern hyperthermic ablative technologies rely on exposure of tumors to supranormal temperatures to ablate intrahepatic tumors. In contrast to cryotherapy, where tumors are more resistant to freezing than normal cells, malignant cells are more sensitive to hyperthermic damage than normal cells. Tumors lack the ability to dissipate heat by augmenting blood flow that is found in healthy tissues.
For most tumors, exposure to temperatures above 42°C results in low-level thermal injury, with increases in injury noted with increased exposure time and temperature. At progressively higher temperatures, there is an exponential and inverse relationship between treatment temperature and the exposure time needed to achieve cell death—that is, higher treatment temperatures require less exposure time for successful ablation. Temperatures above 60°C lead to reliable cell death through complex interactions, involving apoptosis, microvascular damage, ischemia-reperfusion injury, Kupffer cell activation, altered cytokine expression, alterations in the immune response, RNA and DNA destruction, dissolution of lipid bilayers, and protein denaturation. Thermal coagulation begins at approximately 70°C, and tissue desiccation occurs at approximately 100°C.
Radiofrequency Ablation of CLM
RFA achieves local hyperthermia using high-frequency alternating electric current in the radiofrequency range (100–500 kHz). Local hyperthermia results from ionic vibration and frictional heating of surrounding tissues. RFA gained popularity in the 1990s after reports by McGahan and colleagues and Rossi and colleagues and serves as the prototypical ablation platform for most clinicians today.
Radiofrequency current is applied through an electrode that is deployed within the tumor, using ultrasound, CT, or MRI guidance. RFA probes were initially developed as single-needle electrodes but have evolved to multiprobe and internally cooled arrays with attendant increases in treatment volumes and more reliable geometric ablation zones. Several devices are marketed worldwide, and none has emerged as superior with regard to ablation size, reproducibility, or local tumor control.
Percutaneous, laparoscopic, and open surgical approaches have been successfully used for RFA treatment, each with inherent advantages and disadvantages. The choice of approach should be individualized based on tumor anatomy, extent of disease, and patient comorbidities. An open surgical approach to RFA is preferable for patients with large tumors, multiple tumors, and tumors near large blood vessels that may otherwise be inadequately ablated because of heat sink effects. Hepatic inflow occlusion (Pringle maneuver) diminishes the heat sink effect from large intrahepatic vessels and is easier with open surgery compared with laparoscopic surgery. An open approach also allows for extremely accurate intraoperative ultrasound, which facilitates ablation of large tumors near blood vessels or in difficult anatomic regions of the liver. Peripherally situated tumors may be safely ablated by packing adjacent organs away from the liver, thus providing a layer of protection difficult to achieve with other approaches. Thoracic transdiaphragmatic approaches to tumors near the dome of the liver have also been described, and this technique may be useful for patients in whom multiple prior surgical procedures have resulted in prohibitive perihepatic scar tissue.
Laparoscopic RFA is an option for patients with tumors for which percutaneous RFA is not feasible or safe, such as peripherally situated tumors near adjacent organs, such as the stomach or colon. Patients may benefit from the decreased incisional morbidities and faster recovery times afforded by a laparoscopic approach. Substantial surgical judgment must be exercised, however, in selecting patients for whom the laparoscopic approach is appropriate, and oncologic principles should not be compromised intraoperatively. Modern endoscopic optics and laparoscopic ultrasound probes permit excellent visualization, making this approach increasingly attractive.
Percutaneous RFA is well suited for patients with CLM who are not good candidates for more invasive procedures due to comorbid conditions. In general, percutaneous RFA requires tumor sizes and locations that can be accessed without damaging adjacent organs or vascular structures. Although CT-guided RFA is relatively easy for small tumors in the lower segments of the liver, tumors along the edge of the liver can pose exposure challenges. Artificially induced ascites or pleural effusions by injection of dextrose solution has been reported as a means of separating vulnerable nearby organs, thus potentially expanding this indication. Cirrhotic patients and patients with limited intrahepatic recurrences after hepatectomy are examples of patients who may be best served by a percutaneous approach.
Regardless of the device or approach used, attention to RFA probe placement is critical to successful tumor ablation. Careful assessment of preablation imaging studies (CT or MRI) and thorough ultrasound examination of both the tumor and surrounding hepatic anatomy during treatment assist with successful probe placement. Ultrasonography can monitor the progression of the ablation during RFA. Gas bubbles generated by an ablation may interfere with accurate ultrasonography of tissues deep to the electrode, which can hinder repositioning of the electrode for overlapping ablation zones when treating larger tumors. Initial ablation of the deepest portions of the tumor, followed by serial redeployment as the electrode is withdrawn may mitigate this effect. Imaging-related difficulties may be more pronounced with the percutaneous approach, because transcutaneous ultrasound may not be as accurate as intraoperative ultrasonography; however, an immediate post-RFA CT can be readily performed to assist with the assessment of the ablation zone.
Complication rates associated with RFA are low, ranging from 2.4% to 27%, depending on the threshold for defining complications. By compiling data from more than 1300 patients from 18 different studies, Scaife and Curley reported an overall mortality rate of 0.5%, a major complication rate of 2%, and a minor complication rate of 6% after hepatic RFA. The risk of hepatic failure is low after RFA, even in patients with abnormal hepatic parenchyma. For this reason, RFA seems particularly attractive for cirrhotic patients. Monopolar RFA requires careful placement of grounding pads before ablation, because inadequate electrical grounding has been implicated in full-thickness skin burns. Other potential complications after RFA include wound infections, intra-abdominal abscess, renal failure, hepatic abscess, biliary tract injury, pleural effusion, fever, pain, and minor hemorrhage. A postablation syndrome has also been described and is characterized by low-grade fever, malaise, chills, myalgia, delayed pain, and nausea and vomiting. This syndrome is usually self-limited and resolves within 10 days but must be differentiated from more serious postoperative complications. Both RFA and MWA can be safely performed in patients with implanted cardiac devices but require coordination with cardiologists and perioperative device interrogation.
Because there are no published randomized controlled trials comparing RFA and resection, the effectiveness of RFA in CLMs is largely based on several single-arm, retrospective or prospective studies. These studies have inherent flaws (eg, selection bias, differing endpoints, and varying definitions of eligibility for RFA or resection); however, the available data point to RFA as an effective treatment modality in improving survival in CLMs ( Box 1 ). In an American Society of Clinical Oncology clinical evidence review, Wong and colleagues reported wide variability in reported 5-year survival rates (14%–55%) and local recurrence rates (3.6%–60%) after RFA, which are indicative of variability in selection criteria, treatment experience/technique, and endpoints across multiple institutions.