A wide array of ablation technologies, in addition to the progressive sophistication of imaging technologies and percutaneous, laparoscopic, and open surgical techniques, have allowed us to expand treatment options for patients with liver tumors. In this article, technical considerations of chemical and thermal ablations and their application in hepatic oncology are reviewed.
Key points
- •
When choosing an ablation technique or technology, the surgeon should not pit options against each other as a dichotomous choice. Rather, these options should be thought of as tools that the surgeon may select according to the needs of a particular clinical scenario.
- •
Benign and malignant, as well as primary and metastatic tumors may all be ablated in appropriately selected clinical scenarios.
- •
In general, ablation is limited to a few and small tumors and is favored over resection in patients who are poor candidates for major surgical interventions.
- •
Ablation technologies can generally be broken down into chemical and thermal. Each of these technologies can be applied using a percutaneous, laparoscopic, or open surgical approach.
Introduction
Surgical resection has historically been considered the gold standard for curative treatment in patients diagnosed with hepatocellular carcinoma (HCC), cholangiocarcinoma, colorectal cancer hepatic metastasis (CRLM), and selected patients with other types of primary and secondary tumors with liver-only disease. Nonetheless, only 5% to 15% of patients are candidates for liver resection because of tumor size, location, volume, multifocality, or because of inadequate functional hepatic reserve or comorbid disease. Ablative therapeutic techniques have been introduced as safe and effective treatment alternatives to hepatic resection. Recently, randomized trials and case-matched series have indicated that results of ablative techniques can approach equivalence to results of resection in subsets of these patients.
A wide range of ablative technologies and techniques have been introduced over the last several decades, and each has specific advantages relative to the others, depending on the clinical situation. Ablative technologies generally fall into 2 categories: chemical and thermal. More recently, irreversible electroporation (IRE) has been introduced as an alternative form of electrically induced cellular destruction. In this article, a general overview is provided of liver tumor ablation technologies and techniques for use, as well as an overview of technology selection, patient selection, current ablation strategies, and overall outcomes.
Introduction
Surgical resection has historically been considered the gold standard for curative treatment in patients diagnosed with hepatocellular carcinoma (HCC), cholangiocarcinoma, colorectal cancer hepatic metastasis (CRLM), and selected patients with other types of primary and secondary tumors with liver-only disease. Nonetheless, only 5% to 15% of patients are candidates for liver resection because of tumor size, location, volume, multifocality, or because of inadequate functional hepatic reserve or comorbid disease. Ablative therapeutic techniques have been introduced as safe and effective treatment alternatives to hepatic resection. Recently, randomized trials and case-matched series have indicated that results of ablative techniques can approach equivalence to results of resection in subsets of these patients.
A wide range of ablative technologies and techniques have been introduced over the last several decades, and each has specific advantages relative to the others, depending on the clinical situation. Ablative technologies generally fall into 2 categories: chemical and thermal. More recently, irreversible electroporation (IRE) has been introduced as an alternative form of electrically induced cellular destruction. In this article, a general overview is provided of liver tumor ablation technologies and techniques for use, as well as an overview of technology selection, patient selection, current ablation strategies, and overall outcomes.
Ablation technologies
Tumor ablation is not a new concept. Crude versions of tissue or tumor ablation have been reported over the centuries. With a better understanding of oncology and the advent of modern imaging and ablation technologies, we have developed the ability to evaluate tissues harboring cancers and accurately target tumors and safely ablate them. Although the usefulness and efficacy of using these strategies continues to be worked out, there is clear evidence that in selected cases, patients can be cured from lethal diseases with minimal morbidity.
Currently, the most common forms of tumor ablation use either chemical or thermal technologies. Specific details of each are reviewed later. In general, the interest in using nonresective techniques stems from several factors. Although resection has become significantly safer over the last 2 decades, and it is now possible to perform resection using minimally invasive approaches, the incidence of complications associated with ablation is substantially less than that described for surgical resection. In addition, because ablation can be targeted toward individual tumors, surrounding parenchyma is spared, an important factor in patients with extensive disease, limited liver reserve, or who may require repeated interventions.
Chemical Ablation
The most widely used form of chemical ablation is injection of ethanol, although some centers have reported using 5% acetic acid as an alternative. Slow injection of 95% ethanol directly into a tumor induces local coagulation necrosis, thrombosis of tumor microvasculature, and tissue ischemia.
Benefits of chemical ablation include the simplicity of the procedure and the relatively lower cost of the procedure. The chemicals are inexpensive and if the target is easily identifiable, the procedure can be performed under ultrasonographic (US) guidance as an outpatient. A critical advantage of chemical ablation is the ability to limit the collateral damage by targeting tumors immediately adjacent to central biliary and vascular structures, which may be damaged by thermal ablation.
An important limitation of chemical ablation is that it depends on an equal distribution and exposure of the tumor to the chemical ablatant. Although HCC and neuroendocrine tumors (NETs) are relatively soft and often encapsulated, harder tumors such as metastatic adenocarcinoma, typically hard and fibrous, do not allow distribution of the ablatant within the tumor parenchyma.
As a result of relatively high recurrence rates, chemical ablation is typically considered only for patients with small HCCs (<2 cm), who are not resection or thermal ablation candidates (ie, adjacent to central biliary structures).
Thermal Ablation
Cryotherapy
Cryoablation is a technique that uses probe tips that are supercooled by the circulation of either liquid nitrogen or liquid argon. Supercooling to temperatures lower than –170°C induces cell death through the induction of ice crystal formation, cell wall disruption, and microvascular thrombosis.
The advantage of cryotherapy is primarily the ability to easily visualize the lead edge of the ice ball, and, therefore, the margin of the ablation. The lead edge is a bright echo reflector and easily seen on US.
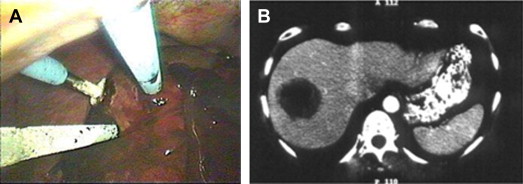
There are several important limitations of cryotherapy. Probes range in diameter from 5 mm to 10 mm. The maximal size of ice ball created using a single probe is 5 cm. To create larger ablation zones, multiple probes are required. During the thaw cycle, ablations are prone to freeze fractures, akin to dropping an ice cube in a glass of warm water. Such freeze fracture can result in vascular injuries and significant hemorrhage. On removal of the probes, hemorrhage within the tract is not uncommon and is typically managed by packing the tract with thrombogenic agents. Both of these issues limit a minimally invasive approach. There are posttreatment systemic effects that are unique to cryotherapy, rarely seen with heat ablation. These effects include severe thrombocytopenia, coagulopathy, myoglobinuria, acute renal failure, as well as electrolyte disturbances and cardiac arrhythmia. These potential problems require additional monitoring and fluid management and prolong postprocedure hospital stay ( Fig. 1 ).
Heat therapy
There are a wide variety of technologies that induce tissue heating to temperatures higher than 50°C. At this temperature, proteins begin to denature, cell walls degrade, and microvascular thrombosis occurs. Higher than 60°C, cells die instantaneously. Thus, any technology that can be applied safely and accurately enough to induce 100% cellular necrosis of the target tumor and spare surrounding tissue may serve as an ablation technology. The most widely available technologies include radiofrequency ablation (RFA) and microwave ablation (MWA).
There are several limitations with current thermal ablation technologies. Thermal ablation depends on achieving lethal temperatures in the entire treatment region. Although measurement of temperature at several points within the ablation zone is available for one of the clinically available thermal devices, others do so at a single point, or by using empirically determined ablation times, or impedance measurements. With tumors located close to central biliary structures, the thermal energy used to destroy the target tumor may also cause irreversible damage to the bile duct, which can lead to abscess or biliary stricture. Also, adjacent viscera, such as duodenum and colon, are easily damaged if near an ablation zone. Most surgeons avoid using thermal ablation within 1 to 2 cm of these structures and are careful to exclude colon and duodenum from the region being treated.
Radiofrequency ablation
RFA is the most commonly used technology for heat ablation. RFA uses a high-frequency alternating current to change the orientation of ions in the electrical field applied to the tumor and surrounding tissue. The agitation of ions creates frictional energy and heat, which are distributed via conduction. Ablation times for a single target depend on size and location, but range between 10 and 30 minutes, with an average of about 20 minutes for a 2-cm tumor. Advantages of RFA include the ease with which the technology can be applied percutaneously, laparoscopically, or in open surgery. The largest commercial probes allow the creation of a 6-cm to 7-cm zone of ablation with the placement of a single probe. This strategy facilitates the ablation of larger lesions with a single needle placement. The disadvantage of RFA is that it is slower than MWA and does not heat large vessels as well as MWA ( Fig. 2 ).
Microwave ablation
MWA uses dielectric hysteresis to produce heat. Tissue destruction occurs when tissues are heated as a result of the application of a magnetic field, typically 900 to 2500 MHz. The polar molecules present in tissue are forced to continuously realign with the oscillating electric field increasing their kinetic energy, increasing the temperature of the tissue. Tissues with a high percentage of water, including tumors, are sensitive to this type of heating.
Microwaves are capable of propagating through and effectively heating many types of tissue, even those with low electrical conductivity, high impedance, or low thermal conductivity. Microwaves can readily penetrate the charred or desiccated tissues that tend to build up around other thermal ablation applicators, resulting in limited power delivery for non–microwave energy systems.
Advantages of MWA include the speed of ablation (range of 5–20 minutes and an average of 12 minutes for a 2-cm tumor) and the ability to treat tumors immediately adjacent to vasculature without the loss of heat as a result of the radiant cooling effect of blood flow. The main disadvantage of the currently available technologies is the size limitations of ablations using single probes. The maximal diameter of a single probe ablation is around 4.6 cm. Thus, the largest target tumor with a 1-cm margin that can be ablated with a single probe application is 2.6 cm. Larger ablations require multiple applications of a single probe or placement of multiple simultaneous probes, both of which may be problematic and raise the possibility of treatment failure, secondary to lack of overlap of concentric treatment zones ( Fig. 3 ).
Irreversible electroporation
IRE is a newer, nonthermal ablation technique that uses pulsed direct current to induce cell death. IRE takes advantage of the electrical gradient that exists across cell membranes, and the application of high-voltage direct electrical current across the cell has the ability to alter the transmembrane potential and disrupt the lipid bilayer. This situation leads to the creation of small nanopores, which allow for an increased influx of extracellular ions. When the voltage applied is sufficiently high, these pores become permanent. These ions are cleared by adenosine triphosphate (ATP)-dependent ion pumps, resulting in intracellular depletion of ATP, which subsequently leads to cell death by apoptosis, with tissues reaching a maximum apoptotic rate after 24 hours. Although cell death is believed to occur via the formation of permanent nanopores in the cell membrane, the extracellular matrix, and thus, collagen structure of vessels and ducts, seem to remain intact.
Unlike thermal ablation techniques, IRE does not encounter the problem of a heat-sink effect, in which local flowing blood draws off the induced heat, with resultant reduced therapeutic effect. IRE is believed to be safe close to vital structures, because the proposed mechanism of action is nonthermal, although more recent articles have pointed out a thermal potential for IRE.
Patient selection
Tumor Types
With the exception of extrahepatic bile duct tumors, essentially all primary and secondary tumor types in the liver are potential candidates for ablation. The indications for tumor ablation are varied and are based on clinical decision making similar to that used for liver tumor resection. Although the morbidity profile and traumatic impact of ablation are typically significantly less than those for resection, the clinical indications are not broadened.
In general, benign tumors of the liver are ablated only if they are symptomatic or growing. There are rarely indications to treat focal nodular hyperplasia and hemangiomas with resection or ablation. Adenomas do have a malignant potential and a propensity to bleed. If there are clinical indications to resect an adenoma, ablation may be considered as an alternative. When ablating a benign lesion, the requirement to ablate surrounding margin of normal tissue is typically relaxed, and thus, larger tumors may be treated with curative intent.
The indications to ablate malignant tumors of the liver are again similar to indications for resection. The advantage to the ablative approach is that the reduced traumatic impact, reduced morbidity profile, and parenchymal sparring with ablation, increase the patient pool in which a potentially curative procedure can be performed.
Hepatocellular carcinoma
There are unique features to treating patients with HCC. First is that most patients with HCC have cirrhosis. Limited hepatic reserve restricts a large percentage of these patients from undergoing curative treatments. The parenchymal sparring effect of ablation allows treatment with significantly less impact on hepatic reserve. HCC is often a soft and encapsulated tumor, thus allowing diffuse infiltration of the tumor with chemical ablatants. This situation makes the chemical ablation an option when treating HCC. HCC is often a multifocal tumor, with separate primaries, intrahepatic metastatic lesions, or satellite lesions near, but not a part of the primary tumor. This multifocality leads to a higher rate of treatment failure when using any form of ablation.
Colorectal cancer hepatic metastasis
CRLM are also amenable to an ablative approach. As a gastrointestinal adenocarcinoma, these tumors are firm, and thus, are not good candidates for chemical ablation. There is an increasing body of literature supporting the use of thermal ablation (primarily RFA and MWA) in patients with CRLM. Ablation can be used alone in patients who are not good candidates for surgical resection, or in combination in patients with extensive or bilobar disease.
Neuroendocrine tumors
Patients with NET are also potentially good candidates for ablation. Generally, liver NETs are metastatic tumors, because there are few primary liver NETs. Some are hormonally active and, therefore, symptomatic. NETs are typically slow growing, but persistent tumors, rarely cured once metastatic to the liver. These patients often require multiple interventions over the course of their disease. Because these are soft tumors, lesions abutting central biliary structures may be treated with chemical ablation.
Other
Ablation of a wide variety of other tumor types has been described in the literature. They include primary lesions such as intrahepatic cholangiocarcinoma, noncolorectal gastrointestinal malignancies, breast cancer, head and neck cancers, thoracic malignancies, and sarcomas. The clinical indications for ablating such lesions must be carefully considered. If there are indications to resect such a tumor, then, ablation may be considered.
General Health
One of the advantages of liver tumor ablation is that there are several approaches, including both percutaneous and surgical. Thus, selection of the appropriate ablation technique for a given patient includes not only consideration of the requirements of the tumor ablation but the ability of the patient to tolerate an intervention.
The percutaneous approach is generally believed to be the least traumatic. Both laparoscopic and open surgical approaches require general anesthesia. The laparoscopic approach is typically performed with only 2 10-mm ports. Although this approach is slightly more traumatic than a percutaneous approach, it is barely so. An open approach requires a laparotomy incision and, therefore, patients selected for such an approach must be able to tolerate this more maximally invasive intervention. Chemical ablations can be performed under sedation, although thermal ablations, because of pain, almost always require either deep sedation or general anesthesia.
Ablation Limitations
Although ablative technologies have opened the door to applying curative treatments to a wider array of patients, there are still clearly limitations on its use. All of the ablation technologies have size limitations. In all tumor types, and with all technologies, there is a higher likelihood of treatment failure in larger tumors. The bulk of the literature suggests that most technologies work well in tumors smaller than 3 cm. The likelihood of treatment failure is intermediate in tumors between 3 cm and 5 cm, and the likelihood of failure in tumors larger than 5 cm is high. Some clinical scenarios allow for multiple ablations of the same tumor to compensate for larger tumor size, but in practical terms, this almost always results in a poorer outcome.
Similarly, tumor number is a relative contraindication. Although no upper limit of tumor numbers has been established for any tumor type or technology, it is generally accepted that the more tumors requiring treatment, the higher the likelihood of both local and regional treatment failure. Challenges with ablations of multiple tumors include visualization of tumors, because most ablation technologies result in some obscuration of imaging of deeper structures. Keeping track of multiple ablations can be problematic both intraoperatively and postoperatively. Larger treatment volumes are associated with higher morbidity rates. Again, although no hard limits have been established, most practitioners do not ablate more than about 30% of the volume of the liver using any given technique. Treatment of larger volumes may require a combination of resection and ablation. Large volumes of ethanol injection result in acute hypotension and posttreatment intoxication.
As described earlier, thermal ablations within 1 to 2 cm of the central biliary structures (right, left, and common hepatic ducts) are fraught with danger. Thermal injury is nondiscriminating, and although larger central blood vessels remain patent because their, blood flow protects them by radiant cooling, the bile ducts are vulnerable. Some practitioners have described using cooling techniques, such as flushing the bile duct with cooled saline during the ablation. The degree of heating is difficult to measure and control accurately. Most practitioners avoid aggressive thermal ablations in this area and instead opt for resection or chemical ablations where indicated ( Fig. 4 ).
Ablation approaches
Percutaneous/Laparoscopic/Open Surgical
Each approach has theoretic and proven advantages and disadvantages. Percutaneous ablation has the advantage of providing a safe and the least invasive and least expensive approach to treating small hepatic tumors. Whether chemical or thermal, these approaches may be performed under sedation. This factor prevents costs and risks associated with general anesthesia.
The percutaneous approach also has the highest treatment failure rate. The reasons for this situation are multifactorial. The percutaneous approach does not include pretreatment staging with peritoneoscopy or intraoperative contact US. This situation leads to treatment failure because of additional undiagnosed hepatic lesions or extrahepatic disease. Further, tumor targeting is more problematic, because US imaging through the abdominal or chest wall limits the approach and degrades the quality of image relative to surface contact US with either a laparoscopic or open US approach.
Computed tomography (CT) or magnetic resonance imaging (MRI) guided ablations are limited because of the appearance of target tumors on these studies in the absence of contrast agents. Although CT and MRI are highly precise in defining tumor and liver anatomy with contrast, needle placement is performed without contrast when tumors are often not directly visible.
Whether using US, CT, or MRI, tumors that are high under the diaphragm may be difficult to reach percutaneously. Also, tumors on the surface of the liver near adjacent organs, which might be injured during thermal ablation, are at risk with nearby ablations. Both of these scenarios are better managed with laparoscopic or open surgical techniques, in which the dome of the liver can be accessed and surrounding viscera can be safely protected ( Fig. 5 ).
The overall morbidity of a laparoscopic approach is not dissimilar to the percutaneous approach, but with the addition of 2 ports, 1 (5–10 mm) for the camera and a 10-mm port for the US device. However, laparoscopy requires general anesthesia and sometimes lysis of adhesions to gain access to the liver. Surgical incisions are sometimes a problem in cirrhotic patients who are at risk for the development of postprocedure liver decompensation and ascetic leaks.
An open approach involves a greater degree of trauma to the patient, with the associated physiologic stresses and recovery. Although there are no segments of the liver that cannot be accessed by a laparoscopic approach, an open approach may facilitate a concomitant procedure, such as a major resection. An open approach may also be useful if the surgeon does not have access to laparoscopic equipment or has not had training in the more technically challenging laparoscopic approach.
Targeting Techniques
Ultrasonographic tumor targeting
The basic technique involves locating the target tumor with the US. The US is aligned such that the ablation device courses parallel to the long axis of the US image as it approaches the tumor. This strategy allows the surgeon to adjust the angle (depth) of approach from top to bottom. By rolling the US slightly side to side, the surgeon can direct the ablation probe side to side to the center of the target.
With open surgical approaches, the entry point of the ablation probe is often close to the end of the US probe. The optimal angle of approach is about 45°. Too steep of an approach makes it difficult to see the approaching ablation probe. Using a laparoscopic technique, selecting the entry point on both the skin and the liver surface can be more challenging, because the surgeon needs to anticipate the thickness of the abdominal wall, the distance to the liver, and the depth of the target within the liver ( Fig. 6 ).
Perioperative process
Preoperative Evaluation
Treatment eligibility and screening generally involve routine history and physical, laboratory tests, and multiphasic cross-sectional imaging with either CT or MRI.
Patient Positioning
Percutaneous
The patient is positioned supine, and a sterile field is created. Heavy sedation or general anesthesia is often required, because thermal ablations can generate a strong pain and distress response. Placement of the ablation probe occurs under image guidance, ensuring that there is a safe pathway and nearby organs are not at risk for injury. After sedation or induction of general anesthesia, the ablation device is deployed into the tumor. Chemical ablatants are injected, monitoring the hypoechoic blush to ensure even disbursement throughout the target. If thermal ablation is being used, the probe is deployed into the tumor; precise placement within the target is required to ensure complete treatment. The ablation is performed, ensuring that the target tumor and a 1-cm margin of normal liver are ablated.
Laparoscopic
The patients is placed supine, general anesthesia is induced, and a sterile field is created. The surgeon stands on the patient’s left, with the laparoscopic and US monitors near the right shoulder. This strategy allows the surgeon to stand in alignment with the target organ and the monitors. One 10-mm port is placed below the margin of the liver, lateral to rectus muscle. One port placed near the umbilicus is used for the camera (5–10 mm). After induction of general anesthesia, trocars are placed into the abdomen, and a staging laparoscopy is completed. In the absence of obvious unresectable extrahepatic disease, intraoperative US is performed to stage the current hepatic disease accurately and to direct treatment. Under US guidance, the RFA probe is deployed into the tumor, and a 1-cm margin of normal liver is ablated ( Fig. 7 ).
Open surgical approach
Generally, a right subcostal or midline incision is made, with the surgeon standing on the patient’s left for the RFA portion of the procedure. The US monitor is again placed near the patient’s right shoulder to facilitate alignment of the surgeon, target tumor, and monitor. After the appropriate incision is made, exploration of the abdomen, and resection of other tumors if applicable, US of the liver is undertaken. Using direct contact US, the ablation device is passed into the liver and target and the ablation performed. Open RFA may facilitate access to the posterior aspects of the right lobe, segments 8 and 7, because ablation angles are more versatile, although experienced laparoscopists can generally reach all 8 segments.
Chemical ablation
The volume of ethanol required to ablate a tumor depends on the size of the tumor, the total dose being based on the results of imaging studies. An injection volume equivalent to the estimated target volume is adequate. For spherical tumors, the formula (4/3*pi*r 2 ) is used. Although pure 95% ethanol is used for smaller volume injections, larger injections may require dilution with saline to as low as a 50% concentration. Large volume injections (>50 mL) must be performed with caution and under careful monitoring, because ethanol injections can cause transient hypotension and intoxication.
The injection is started on the deep aspect of the tumor, because the injectate creates a hyperechoic field, obscuring imaging of deeper structures. Injection may be performed with standard 15.2-cm (6 in), 20-gauge to 22-gauge spinal needle, or using a specially designed conical-tip needle with multiple side holes. The position of the needle is moved around inside the tumor during the injection to ensure an even distribution of injectate throughout the target.
Postoperative Concerns
Percutaneous and laparoscopic thermal ablation
Patients who undergo CT-guided ablations are typically discharged from the short-stay unit within 23 hours of the procedure, although some procedures are performed with same day discharge.
After laparoscopic RFA, patients are generally kept overnight for observation and discharged on postoperative day 1. Same day discharge is becoming more common. Before discharge, patients must be ambulatory, tolerate oral fluid intake, have adequate pain control, and be reliable and willing to return if problems arise. We recommend overnight observation of most patients with intrinsic liver function compromise, or patients with portal hypertension.
Open thermal ablation
Generally, length of hospital stay for patients with open RFA is determined by pain control and postlaparotomy factors. Liver function should be assessed.
Complications and Management
Complications after ablation are uncommon (1%–5%). Although large volume heat ablations can induce intermittent fevers related to volume of tissue destruction, the procedure is generally well tolerated. Most patients have pain in the epigastrium, right upper quadrant, and right flank, which can last 1 to 2 weeks. Liver abscesses can occur, primarily in patients who have had previous biliary manipulation. Bleeding and bile leak occur rarely. More rare, but more serious complications have been reported and include injury to bowel or bile-pleural fistulae ( Fig. 8 ).






