Increasing data on treatment of liver metastases with locoregional therapies have solidified the expanding role of interventional radiologists (IRs) in the treatment of liver metastases from colorectal cancer. Ablative approaches such as radiofrequency ablation and microwave ablation have shown durable eradication of tumors. Catheter-directed therapies such as transarterial chemoembolization, drug-eluting beads, yttrium-90 radioembolization, and intra-arterial chemotherapy ports represent potential techniques for managing patients with unresectable liver metastases. Understanding the timing and role of these techniques in multidisciplinary care of patients is crucial. Implementation of IRs for consultation enables better integration of these therapies into patients’ overall care.
Key points
- •
Interventional radiologists play an important role in the multidisciplinary care of patients with colorectal cancer with liver metastases.
- •
Ablation therapy can focally destroy liver metastases, providing long-term survival in select cases.
- •
The “test-of-time” approach with ablation before hepatic metastatectomy can help select patients who will benefit from surgery and patients whose biology will lead to innumerable metastases, making them ultimately not a good surgical candidate.
- •
Intra-arterial therapies with chemoembolization, radioembolization, drug-eluting beads, and intra-arterial chemotherapy can play a role in patients with unresectable liver metastases and liver-dominant disease.
Introduction
Treatment of limited metastatic disease can prolong survival; however, in many instances patients may not be ideally suited for surgery, may not be able to discontinue chemotherapy in the perioperative period, or may prefer a less invasive therapy to maintain quality of life. Several image-guided procedures have evolved within the last 10 years that allow the management of limited metastatic disease in nonresectable patients and those that have recurred after resection. These locoregional therapies include local tumor ablation and catheter-directed therapies, and can occasionally downstage a patient with inoperable metastases to an operable status. Ablative therapies cause focal destruction of tissue, and have increasingly been shown to successfully eradicate colorectal liver metastases. Although it has been less used than hepatic ablation, pulmonary thermal ablation has achieved promising results with respect to safety and local tumor control. An increasing number of studies evaluating local ablation for the treatment of pulmonary colorectal metastases is emerging. Definitive integration of ablative therapies into the management of metastatic colorectal cancer depends on defining the patient population in which ablation can be used instead of surgery with equivalent results. In hepatocellular carcinoma, for example, the data have suggested that for tumors smaller than 2 cm ablation may be preferable to surgery. With improving technology, similar equivalencies with ablation in colorectal metastases may arise. Catheter-directed therapies such as transarterial chemoembolization (TACE), drug-eluting beads (DEB), selective internal radiation therapy (SIRT), and intra-arterial chemotherapy ports are all potential techniques for managing the patient with unresectable liver metastases.
Introduction
Treatment of limited metastatic disease can prolong survival; however, in many instances patients may not be ideally suited for surgery, may not be able to discontinue chemotherapy in the perioperative period, or may prefer a less invasive therapy to maintain quality of life. Several image-guided procedures have evolved within the last 10 years that allow the management of limited metastatic disease in nonresectable patients and those that have recurred after resection. These locoregional therapies include local tumor ablation and catheter-directed therapies, and can occasionally downstage a patient with inoperable metastases to an operable status. Ablative therapies cause focal destruction of tissue, and have increasingly been shown to successfully eradicate colorectal liver metastases. Although it has been less used than hepatic ablation, pulmonary thermal ablation has achieved promising results with respect to safety and local tumor control. An increasing number of studies evaluating local ablation for the treatment of pulmonary colorectal metastases is emerging. Definitive integration of ablative therapies into the management of metastatic colorectal cancer depends on defining the patient population in which ablation can be used instead of surgery with equivalent results. In hepatocellular carcinoma, for example, the data have suggested that for tumors smaller than 2 cm ablation may be preferable to surgery. With improving technology, similar equivalencies with ablation in colorectal metastases may arise. Catheter-directed therapies such as transarterial chemoembolization (TACE), drug-eluting beads (DEB), selective internal radiation therapy (SIRT), and intra-arterial chemotherapy ports are all potential techniques for managing the patient with unresectable liver metastases.
Ablative therapy for colorectal liver metastases
Hepatectomy with curative intent is the treatment of choice for colorectal liver metastases (CLM), although only 20% of patients are resectable at presentation. Five-year survival rates after complete resection of CLM range from 35% to 58%. For unresectable patients, median survival ranges from 6 months without any treatment to 12 to 24 months for patients receiving first-line and second-line chemotherapy. Ablation technologies cause focal destruction of tissue. Several ablative tools are currently used for the treatment of colorectal cancer metastases, including radiofrequency ablation (RFA), microwave ablation (MWA), cryoablation, laser ablation, and focused ultrasound ablation, all of which rely on extreme heat or cold to produce cell death and exact tissue damage. A nonthermal ablation technique, irreversible electroporation (IRE), uses electrical fields to cause cell death without apparently harming tissue protein architecture that makes up structures such as bile ducts and vessels. This newer technique may open up new ablative opportunities near critical structures that would otherwise not be possible to ablate safely with thermal energy.
The advantages of ablation techniques derive from their being less invasive and, consequently, less morbid than surgical resection. Patients can generally be treated as outpatients, with a rapid recovery to normal activities. Ablative therapies minimize the destruction of healthy liver tissue in comparison with hepatic resection. This parameter is an important one for patients with underlying cirrhosis or steatohepatitis from prolonged chemotherapy exposure, and those who have previously undergone extensive liver resection. Percutaneous ablation procedures can be repeated to treat recurrences as needed, with minimal loss of unaffected parenchyma. As such, ablation can be used to salvage some recurrences after resection. Whereas chemotherapy routines are frequently interrupted for 6 weeks by surgical resection, this same requirement is not present with percutaneous ablation techniques.
RFA is the most widely used and well-studied ablation modality. RFA has been used to control CLM successfully in selected patients with nonresectable disease. There is an overall recognition that for small tumors that can be ablated completely with clear margins, RFA may be able to provide results similar to those of surgery, despite the fact that ablation series included patients with major medical comorbidities, insufficient hepatic reserve for surgery, extrahepatic disease, or tumors located in proximity to major vascular structures. In addition, the long survival times after curative surgery may reflect selection bias of early detection of CLM. These results support the use of thermal ablation as a procedure with curative intent, similarly to surgical metastasectomy, for selected patients with small size and number of CLM that can be ablated with appropriate margins. To date, a randomized study for resectable liver metastases comparing resection with RFA has not been completed.
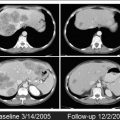
A key component of a successful ablation is the creation of an effective ablation zone that will encompass the target lesion with clear margins (ideally 1 cm all around the target tumor). Imaging guidance during the procedure is critical to ensure adequate targeting and evaluate for completeness of the ablation ( Fig. 1 ). The ablation zone should not enhance, and should be larger than the target tumor accounting for the desired margin.
Median overall survival (OS) times beyond 30 months after thermal ablation are superior when compared with unresectable historical controls treated by chemotherapy, but these findings can be biased by patient selection. However, it is not uncommon that patients undergo thermal ablation only after they have failed several lines of chemotherapy. Prospective randomized trials are essential in this case as well, to evaluate the exact benefit of tumor ablation therapies over systemic chemotherapy. As yet there is a single randomized clinical trial comparing RFA combined with systemic chemotherapy with systemic chemotherapy alone as first-line therapy for CLM. The benefit of RFA in combination with systemic chemotherapy is reflected in a statistically significant prolongation of median progression-free survival (PFS) to 16.8 months for the combined arm, compared with 9.9 months for the chemotherapy-only arm. Overall patient survival was 61.7% for the combined therapy and 57.6% for the chemotherapy-only control group at 30 months. This modest prolongation did not reach significance in a study that was not powered to address this and with a follow-up time of only 30 months.
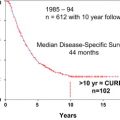
Results from long-term follow-up (up to 10 years) are now available from a retrospective study. These results (5-, 7-, and 10-year OS rates of 47.8%, 25%, and 18%, respectively) are essentially equivalent to those of surgical resection. Survival was significantly improved in patients who underwent repeat ablation after local tumor progression (45.5 months vs 31 months for patients who were not retreated).
The local recurrence rate after RFA varies widely between 2% and 60%, and remains an important limitation of RFA, especially when compared with resection. Size of the lesion, location from major vessels, and ablation margin are key factors that have been associated with local tumor control. A tumor size greater than 3 cm is now known to be associated with a higher rate of local progression after RFA. Tumors adjacent to vessels larger than 3 mm have an increased risk of local recurrence, because thermal energy is dissipated away from the target tumor as a result of adjacent blood flow (the so-called heat-sink phenomenon). Like the surgical margin, the size of the ablation margin has been associated with local tumor control.
Cryotherapy alone or in combination with hepatic resection has been used in selected patients, with oncologic outcomes comparable with surgery. Cryoablation applied to the edge of hepatic resections (edge cryotherapy) is frequently used in cases with “close” margins, affording a 5-year survival rate of 31% for advanced colorectal cancer, as reported by Ng and colleagues. The same investigators reported a 5-year survival rate of 21% for the group treated with hepatic cryotherapy alone or in combination with liver resection, with curative intention. These results are promising, and suggest that this method can afford a potential cure for selected cases of advanced CLM not amenable to curative surgery with hepatectomy alone.
Test of Time
In selected resectable patients, percutaneous ablation was proposed with the concept of the “test of time.” Using this approach, selected resectable patients with small-volume disease that can be ablated with margins underwent percutaneous ablation while being observed and imaged, with the intention to undergo hepatic metastasectomy. This delayed period allows the biology of disease to express itself. Patients completely treated by ablation without local recurrence, in addition to those who develop multifocal unresectable metastases, are spared unnecessary surgery. For patients with local recurrence or limited intrahepatic progression, there would still be an opportunity to repeat ablation or undergo metastasectomy. The test-of-time approach could represent the starting point in prospective randomized trials comparing percutaneous ablation with surgery that will support the use of ablation as the first-line treatment of CLM in selected cases. One other practical application of the test-of-time approach is for the treatment of recurrent CLM after hepatic resection (particularly new liver metastasis within 6 months of resection) in patients who are technically re-resectable. Patients with successful ablation and those who would have further recurrences benefit from avoiding the increased morbidity of nonbeneficial re-resection.
Newer Technologies: Microwave Ablation and Irreversible Electroporation
MWA was developed to overcome some of the limitations of RFA. MWA may provide larger and more uniform ablation volumes in a shorter time and higher rates of complete ablation, owing to avoidance of the heat-sink effect. MWA has demonstrated its superiority over RFA in treating larger tumors, resulting in lower recurrence rates (as low as 6%). With regard to OS, intraoperative MWA has produced results similar to those of surgery in retrospective series, up to a median of 43 months. To date there is a single randomized trial comparing microwave ablation with hepatic resection in patients with CLM. The mean survival time was 27 months in the MWA group, compared with 25 months in the surgery group. The mean disease-free interval was 11.3 months in the MWA group versus 13.3 months in the surgery group. These differences were not statistically significant. This trial was underpowered to determine differences in survival between the groups, owing to the small sample size (40 patients, from which 10 were excluded after randomization).
IRE is generally indicated for tumors that are deemed inappropriate for thermal ablation because of the relatively high risk of collateral damage caused by tumor proximity to bile ducts or other structures that would be injured by the heat. Clinical series that included patients with CLM have demonstrated a primary efficacy of 67% to 100% for tumors adjacent to major vascular/biliary structures. The risk of recurrence seems to be higher for tumors greater than 3 cm in size. Current evidence, while encouraging, is still limited, with no randomized controlled trials. Most of the series included different organs and differing pathology, making it difficult to draw any generalized conclusions.
Catheter-directed liver therapies
Arterial therapies for colorectal liver cancer metastases can be performed to complement or salvage the effects of systemic therapy. The concept of intra-arterial therapies relies on the fact that liver cancers derive their blood supply predominantly from hepatic arteries, whereas normal liver parenchyma has a predominantly portal vein source of blood supply. The most common techniques of intra-arterial therapies for CLM include intra-arterial hepatic chemotherapy (IAHC), TACE, and SIRT with yttrium-90–impregnated microspheres. Two more therapies are available, namely intraportal drug delivery and isolated liver perfusion.
Intra-arterial Hepatic Chemotherapy
IAHC aims to increase the drug concentration in liver metastases and thereby improve response rates. This approach can be best applied with drugs having a high first-pass effect. One drug that has been extensively used is floxuridine (FUDR), which has a first-pass extraction rate of 95% and can increase the liver dose by 100 to 300 times higher than the systemic perfusion. Historically, repeated or continuous IAHC has been delivered by a catheter and pump system requiring laparotomy. More recently, IAHC has become deliverable via an interventional radiology approach with a subcutaneous port placed. In one study of 36 patients with extensive nonresectable liver metastases (ie, ≥4 metastases in 86% and bilobar in 91%) using IAHC with oxaliplatin (100 mg/m 2 in 2 hours) plus intravenous 5-fluorouracil (5-FU)/leucovorin (LV) (400 mg/m 2 in 2 hours; 5-FU 400 mg/m 2 bolus then 2500 mg/m 2 in 46 hours) and cetuximab (400 mg/m 2 then 250 mg/m 2 /wk or 500 mg/m 2 every 2 weeks) as first-line treatment overall response rate was 90% and disease control rate was 100%. Forty-eight percent of patients were downstaged enough to undergo an R0 resection and/or RFA.
Conventional Transarterial Chemoembolization and Transarterial Chemoembolization with Drug-Eluting Beads
There are several different techniques under the acronym TACE. The most common procedure is the intra-arterial injection of chemotherapy emulsified with Lipiodol Ultra-Fluid (LUF; Laboratoire Guerbet, Aulnay, France) followed by injection of embolic material. With Lipiodol-TACE, the ratio of drug concentration in the tumor compared with the healthy liver and peripheral blood levels can be as high as 10 and 1000 times, respectively. Embolization after chemo-Lipiodol increases the efficacy of treatment by prolonging contact of chemotherapy to the tumor cells and by adding ischemia to the highly hypervascularized tumor usually targeted with this treatment. Such embolization has been reported to induce failure of the transmembrane pump, thus increasing drug retention inside the cells.
One group using the regimen of cisplatin, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcohol has shown an overall response rate of 43%. Median survivals of 33 months from initial diagnosis, 27 months from the time of liver metastases, and 9 months from the start of chemoembolization were documented, suggesting a possible improvement over reported survival time for systemic therapies alone. Another group using mitomycin C alone (52.5%), mitomycin C with gemcitabine (33%), or mitomycin C and irinotecan (14.5%) has shown an overall response rate of 63%.
Recently, drug-eluting beads have been developed that allow drug release after the bead has been embolized into the tumor microcirculation. One of the drugs that has been loaded on these beads is irinotecan. The advantage of the beads is a reduced systemic delivery of chemotherapy. Irinotecan-loaded beads had a 75% reduced systemic plasma level compared with intra-arterial irinotecan alone.
In a randomized study of 2 courses of DEBIRI (Biocompatibles, Oxford, CT, USA) (36 patients) compared with 8 courses of intravenous irinotecan, 5-FU, and leucovorin (FOLFIRI; 38 patients) used to treat 74 patients who failed at least 2 lines of chemotherapy, the DEBIRI arm was met with statistically significant improvement of all oncologic outcomes including patient survival. Specifically, the response rates were 69% for the DEBIRI group compared with 30% for the systemic FOLFIRI group. Similarly, the 2-year OS was 56% compared with 32%, and the median OS was 22 months compared with 15 months for DEBIRI versus FOLFIRI groups. Improvement in quality of life was of longer duration for the DEBIRI group (8 months) than for the FOLFIRI group (3 months, P = .0002). Finally, overall cost was lower for the DEBIRI treatment arm.
In a multicenter, single-arm study of 55 patients who underwent DEBIRI after failing systemic chemotherapy, response rates were 66% at 6 months and 75% at 12 months, with an OS of 19 months and a PFS of 11 months.
A recent comparison study of DEBIRI versus radioembolization for salvage therapy for liver-dominant CLM including a series of 36 patients reported similar survival for both treatments, with median survival times of 7.7 months for the DEBIRI group and 6.9 months for the radioembolization (SIRT) group. The 1-, 2-, and 5-year survival rates were 43%, 10%, and 0% in the DEBIRI group and 34%, 18%, and 0% in the SIRT group.
Approximately 20% of DEBIRI sessions are associated with adverse events (most commonly CTCAE [Common Terminology Criteria for Adverse Events] grades 1 and 2) during or after the treatment. The factors predictive of adverse events and significantly longer stay in hospital are: lack of pretreatment with hepatic arterial lidocaine ( P = .005); greater than 3 treatments ( P = .05); achievement of complete stasis ( P = .04); treatment with greater than 100 mg DEBIRI in 1 session ( P = .03); bilirubin greater than 2.0 μg/dL with greater than 50% liver replaced by tumor ( P = .05).
Radioembolization/Selective Internal Radiation Therapy
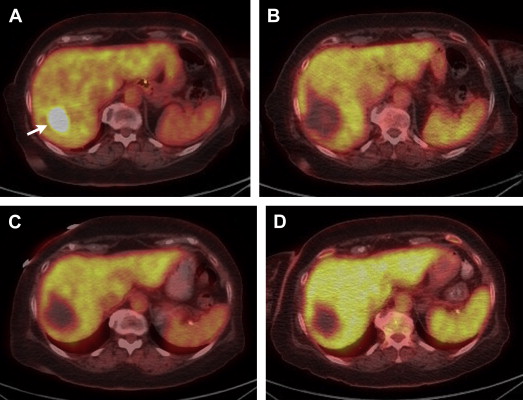
Traditional external beam radiation therapy in patients with diffuse hepatic malignancy does not improve OS because liver tolerance for developing radiation-induced injury is low compared with the doses required for tumoricidal effect. The usual dose to treat a tumor is 70 Gy, whereas normal liver tissue tolerance to radiation is 30 Gy. These facts resulted in the idea of selective internal transarterial radiotherapy with the delivery of yttrium-90–impregnated microspheres. Selective intra-arterial delivery enables doses higher than 120 Gy to target the tumor without reaching the liver toxicity threshold. Radioembolization allows delivery of high doses of ionizing radiation to the tumor with minimal radiation to surrounding tissue, thus causing considerably less toxicity to the normal liver. Patients referred to radioembolization have unresectable (and noneligible for ablation) CLM, liver-only or liver-dominant disease, life expectancy of at least 3 months, and acceptable liver reserve. SIRT has been safely used as a salvage therapy in heavily pretreated patients who progressed after multiple lines of systemic and hepatic arterial chemotherapy in addition to resection. Overall response of 17% to 35% and stable disease rates of 24% to 61% have been described. Median survival after radioembolization has ranged from 6.7 to 17 months. Modest effects of radioembolization were seen when it was used as a salvage monotherapy after complete failure of chemotherapy. Radioembolization alone in this setting showed an overall response of 24%, a PFS of 3.7 months, and 1- and 2-year overall survival rates of 50.4% and 19.6%, respectively. The major contribution of radioembolization was documented when it was used together with systemic chemotherapy ( Fig. 2 ). The major concept behind this combined treatment was that tumors were sensitized by one treatment for the other and, thus, a synergistic effect of SIRT with chemotherapy was seen, with better response rates. In a randomized controlled trial the combination of SIRT with protracted 5-FU had a significantly better PFS when compared with protracted 5-FU alone in patients who had previously failed regimens containing 5-FU.