Metastatic disease is the most common neoplasm involving the skeletal system and can result in significant pain and morbidity. Although narcotic medications and external beam radiation therapy remain the standard of care, several image-guided ablation techniques have evolved to play a role in the management of painful bone metastases. This article reviews the percutaneous ablation techniques available for relieving bone pain in patients suffering from cancer.
Osseous metastases are a common problem for patients with cancer; up to 85% of patients with breast, prostate, and lung cancer have evidence of bone metastases at the time of death. Although most bone metastases are asymptomatic, osseous metastases are a common cause of cancer-related pain and can be quite debilitating, affecting patients’ quality of life, performance status, and mood.
A multidisciplinary team approach with collaborative efforts between medical oncologists, radiation oncologists, surgical oncologists, and interventional radiologists is often necessary to determine optimal therapy as various treatment modalities are available for the management of osseous metastases. Treatments for painful bone metastases are usually palliative. Surgery is generally reserved for stabilization purposes and has a limited role in the palliation of painful bone metastases without pathologic fracture. Systemic therapies (chemotherapy, hormonal therapy, radiopharmaceuticals, and bisphosphonates) have specific yet limited usefulness because painful osseous metastatic disease is usually refractory to standard chemotherapy and hormonal therapy. Analgesics (opioids and nonsteroidal antiinflammatory drugs) remain a cornerstone treatment for many patients with painful bone metastases. However, to achieve adequate levels of pain control, higher doses of these medications are frequently required and can lead to significant side effects, including constipation and over sedation.
External beam radiation therapy (XRT) is the current standard of care for the palliation of patients with cancer with painful bone metastases. However, published reports in the literature indicate that many patients do not achieve optimal relief, with up to 30% of patients experiencing inadequate analgesia following XRT. Moreover, the onset of pain relief is often delayed for up to 4 to 12 weeks following therapy and the durability of pain relief can be short lived with many patients experiencing recurrent pain within months of XRT therapy. Unfortunately, previously irradiated sites are often not eligible for further XRT because of the limitations of normal tissue tolerance.
Image-guided percutaneous ablation techniques have several advantages that address the limitations of XRT and have become an important palliative treatment option in patients with painful bone metastases. The most commonly used methods are radiofrequency ablation (RFA) and cryoablation. The purpose of this article is to summarize the techniques for image-guided ablation of bone metastases and to review the literature on its use in the palliation of bone pain.
Patient selection and preprocedure management
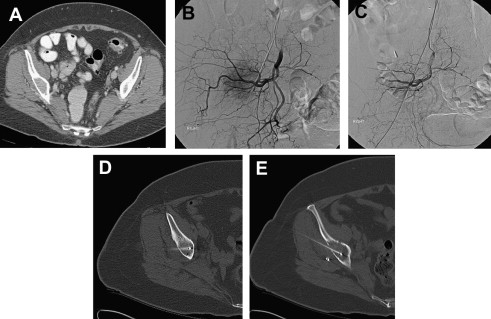
In the palliative setting, ablative techniques are indicated for patients with painful osteolytic bone metastases who are not suitable candidates for or have failed other standard forms of therapy. Although there is no limit to the size of the tumor that can be treated, patients’ pain must be limited, from 1 or 2 sites of metastatic disease in bone, and should rate greater than or equal to 4 on a pain scale of 0 to 10. Contraindications include tumors that are in direct contact with hollow viscera or neural elements. In general, the edge of the ablation zone should be at least 1 cm away from the spinal cord, major motor nerves, brain, artery of Adamkiewicz, bowel, or bladder. Lesions in weight-bearing bones should be treated only if there is no risk of an impending fracture. Patients with recurrent tumors at sites that have been surgically stabilized with metallic hardware are not candidates for RFA but may be eligible for cryoablation. Hypervascular bone metastases larger than 3 or 4 cm may be more suitable for cryoablation because perfusion-mediated tissue cooling may prevent effective RFA ablation. However, preablation arterial embolization of these large, hypervascular tumors can be performed to address this problem and to potentiate the effects of RFA ( Fig. 1 A, B, C, D, E). Finally, treatment of osteoblastic lesions is infrequently performed because of the often difficult access through sclerotic bone and poor RFA energy deposition in these lesions.
Before any image-guided intervention, adequate imaging of the affected site must be obtained for procedure planning. In particular, cross-sectional imaging with computed tomography (CT) or MRI is necessary to assess the extent of disease and the proximity of the lesion to vital structures. Patients should be examined before the procedure to determine the location and severity of the pain. Validated pain scales, such as the Brief Pain Inventory and the Memorial Pain Assessment Card (MPAC), are useful tools for scoring pain severity and provide baseline data for outcome analysis. Lastly, patients’ opioid analgesic use should be inventoried and translated to a morphine-equivalent dose for future comparison.
The platelet count should be greater than 75,000/μL and coagulopathies should be corrected. Anticoagulation medications, such as aspirin, antiplatelet medications, low molecular weight heparin preparations and warfarin, should be discontinued before the procedure.
Methods of ablation
Several image-guided thermal ablation technologies have been developed and used in the ablation of painful bone metastases, including RFA, cryoablation, microwave ablation, laser interstitial thermal therapy, plasma-mediated RF (coablation), and focused ultrasound therapy ; however, this review focuses on the most studied and most commonly used ablation methods, which are RFA and cryoablation. Although the exact mechanism of pain relief following ablation is unclear, it is likely multifactorial and several theories have been put forth. RFA and cryoablation may contribute to relief of tumor-related bone pain by: (1) destroying local sensory nerves supplying the periosteum thereby inhibiting pain transmission, (2) reducing tumor volume thus decreasing the stimulation of sensory nerve fibers, (3) destroying cytokine-producing tumor cells, and (4) inhibiting osteoclast activity.
A summary of the reported studies regarding the use of thermal ablative techniques in the palliative treatment of pain related to osseous metastatic disease is provided in Table 1 . Generally, most studies demonstrate that percutaneous ablative techniques used in this setting are safe and achieve durable palliation in this cohort of patients with limited life expectancy and pain refractory to standard therapies. Nevertheless, extrapolation of these results is limited because of the different parameters used to objectively assess patients’ pain, the heterogeneity of lesions treated (location, pathology, and size), different ablative techniques used (RFA vs cryoablation), and the length of follow-up. Reporting standards and guidelines should be followed to adequately compare trials in the future.
| Author | N | Ablation Method | Prior Treatment | Tumor Size (mean or range) | Pain Assessment Scale | Pretreatment Pain Score | Posttreatment Pain Score | P -Value | Study Follow-Up Period | Additional Comments | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Worst | Baseline | Worst | |||||||||
| Dupuy et al 2010 | 55 | RFA | NR | 5.2 cm | mMPAC | 54.4 | 91.0 | 14.2 | NR | 0.02 | 3 months | — |
| Carrafiello et al 2009 | 10 | RFA/plasma mediated RF ± cementoplasty | 100% | 1.4–8.8 cm | VAS | 8.0 | NR | 2.0 | NR | <0.001 | 3 months | MED decreased from 80–100 to 30–40 at 3 months ( P < .001) |
| Tuncali et al 2007 | 10 | Cryoablation | NR | 5.2 cm | NR | NR | NR | NR | NR | NR | 1–44 weeks (mean 8.9) | 17 of 19 pts had pain palliation (6 of 17 complete, 11of 17 partial) |
| Callstrom et al 2005 | 14 | Cryoablation | 100% | 4.3 cm (1.0–11.0 cm) | BPI, VAS | 4.5 | 6.7 | 2.0 | 3.4 | 0.003 | 8 weeks | No statistically significant change in MED |
| Poggi et al 2003 | 5 | RFA | 100% | 3.0 to 8.0 cm | VAS | NR | NR | NR | NR | NR | NR | 4 of 5 pts had pain palliation (1 pt free up to 88 weeks) |
| Goetz et al 2002 | 43 | RFA | 77% | 6.3 cm | BPI-sf | 5.8 | 7.9 | 1.2 | 1.4 | 0.005 | 24 weeks | No statistically significant change in MED |
| Callstrom et al 2002 | 12 | RFA | 100% | 4.9 cm (1.3–10.8 cm) | BPI, VAS | 6.5 | 8.0 | 1.4 | 2.4 | <0.04 | 1–24 weeks (mean 8) | 8 of 10 pts reduced analgesic medication post-RFA |
| Gronemeyer et al 2001 | 10 | RFA | 100% | 1.5 to 9.0 cm | VAS | 59.5 | NR | 26.0 | NR | NR | 2–11 months | — |
Methods of ablation
Several image-guided thermal ablation technologies have been developed and used in the ablation of painful bone metastases, including RFA, cryoablation, microwave ablation, laser interstitial thermal therapy, plasma-mediated RF (coablation), and focused ultrasound therapy ; however, this review focuses on the most studied and most commonly used ablation methods, which are RFA and cryoablation. Although the exact mechanism of pain relief following ablation is unclear, it is likely multifactorial and several theories have been put forth. RFA and cryoablation may contribute to relief of tumor-related bone pain by: (1) destroying local sensory nerves supplying the periosteum thereby inhibiting pain transmission, (2) reducing tumor volume thus decreasing the stimulation of sensory nerve fibers, (3) destroying cytokine-producing tumor cells, and (4) inhibiting osteoclast activity.
A summary of the reported studies regarding the use of thermal ablative techniques in the palliative treatment of pain related to osseous metastatic disease is provided in Table 1 . Generally, most studies demonstrate that percutaneous ablative techniques used in this setting are safe and achieve durable palliation in this cohort of patients with limited life expectancy and pain refractory to standard therapies. Nevertheless, extrapolation of these results is limited because of the different parameters used to objectively assess patients’ pain, the heterogeneity of lesions treated (location, pathology, and size), different ablative techniques used (RFA vs cryoablation), and the length of follow-up. Reporting standards and guidelines should be followed to adequately compare trials in the future.
| Author | N | Ablation Method | Prior Treatment | Tumor Size (mean or range) | Pain Assessment Scale | Pretreatment Pain Score | Posttreatment Pain Score | P -Value | Study Follow-Up Period | Additional Comments | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Worst | Baseline | Worst | |||||||||
| Dupuy et al 2010 | 55 | RFA | NR | 5.2 cm | mMPAC | 54.4 | 91.0 | 14.2 | NR | 0.02 | 3 months | — |
| Carrafiello et al 2009 | 10 | RFA/plasma mediated RF ± cementoplasty | 100% | 1.4–8.8 cm | VAS | 8.0 | NR | 2.0 | NR | <0.001 | 3 months | MED decreased from 80–100 to 30–40 at 3 months ( P < .001) |
| Tuncali et al 2007 | 10 | Cryoablation | NR | 5.2 cm | NR | NR | NR | NR | NR | NR | 1–44 weeks (mean 8.9) | 17 of 19 pts had pain palliation (6 of 17 complete, 11of 17 partial) |
| Callstrom et al 2005 | 14 | Cryoablation | 100% | 4.3 cm (1.0–11.0 cm) | BPI, VAS | 4.5 | 6.7 | 2.0 | 3.4 | 0.003 | 8 weeks | No statistically significant change in MED |
| Poggi et al 2003 | 5 | RFA | 100% | 3.0 to 8.0 cm | VAS | NR | NR | NR | NR | NR | NR | 4 of 5 pts had pain palliation (1 pt free up to 88 weeks) |
| Goetz et al 2002 | 43 | RFA | 77% | 6.3 cm | BPI-sf | 5.8 | 7.9 | 1.2 | 1.4 | 0.005 | 24 weeks | No statistically significant change in MED |
| Callstrom et al 2002 | 12 | RFA | 100% | 4.9 cm (1.3–10.8 cm) | BPI, VAS | 6.5 | 8.0 | 1.4 | 2.4 | <0.04 | 1–24 weeks (mean 8) | 8 of 10 pts reduced analgesic medication post-RFA |
| Gronemeyer et al 2001 | 10 | RFA | 100% | 1.5 to 9.0 cm | VAS | 59.5 | NR | 26.0 | NR | NR | 2–11 months | — |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree