Chemical ablation
– Ethanol injection
Thermal ablation
– Radiofrequency ablation
– Microwave ablation
– Laser ablation
– Cryoablation
Irreversible electroporation
2 Chemical Ablation
The seminal technique used for local ablation of HCC has been percutaneous ethanol injection (PEI). Ethanol induces coagulation necrosis of the lesion as a result of cellular dehydration, protein denaturation, and chemical occlusion of small tumor vessels. PEI is a well-established technique for the treatment of small, nodular-type HCC [6]. HCC nodules have a soft consistency and are surrounded by a firm cirrhotic liver. Consequently, injected ethanol diffuses within them easily and selectively. The major limitation of PEI is the high local recurrence rate, that may reach 33 % in lesions smaller than 3 cm and 43 % in lesions exceeding 3 cm [7, 8]. The injected ethanol does not always accomplish complete tumor ablation because of its inhomogeneous distribution within the lesion—especially in presence of intratumoral septa—and the limited effect on extracapsular cancerous spread. The recent introduction of a specific device for single-session PEI, a multi-pronged needle with three retractable prongs, each with four terminal side holes (QuadraFuse; Rex Medical, Conshohochen, PA), has been shown to overcome some of these limitations, by ensuring a more homogeneous ethanol perfusion throughout the whole tumor mass. In two recent studies, PEI performed with multi-pronged needles resulted in rates of sustained complete response ranging 80–90 % in tumors smaller than 3–4 cm in diameter [9–11]. Hence, the technique seems still to be able to offer a valuable alternative to RFA, particularly for small lesions in unfavorable location for thermal ablation.
3 Thermal Ablation
The thermal ablative therapies involved in clinical practice can be classified as either hyperthermic treatments—including radiofrequency ablation (RFA), microwave ablation (MWA), and laser ablation—or cryoablation. The thermal damage caused by heating is dependent on both the tissue temperature achieved and the duration of heating. Heating of tissue at 50–55 °C for 4–6 min produces irreversible cellular damage. At temperatures between 60 and 100 °C near immediate coagulation of tissue is induced, with irreversible damage to mitochondrial and cytosolic enzymes of the cells. At more than 100–110 °C, tissue vaporizes and carbonizes [12]. On the other hand, the freezing of tissue with temperatures between −20 and −60 °C followed by rapid thawing results in cell membrane disruption and induces cell death. For adequate destruction of tumor tissue, the entire target volume must be subjected to cytotoxic temperatures.
3.1 Radiofrequency Ablation
The goal of RFA is to induce thermal injury to the tissue through electromagnetic energy deposition. In the more popular monopolar mode, the patient is part of a closed-loop circuit, that includes a RF generator, an electrode needle, and a large dispersive electrode (ground pads). An alternating electric field is created within the tissue of the patient. Because of the relatively high electrical resistance of tissue in comparison with the metal electrodes, there is marked agitation of the ions present in the target tissue that surrounds the electrode, since the tissue ions attempt to follow the changes in direction of alternating electric current. The agitation results in frictional heat around the electrode. The discrepancy between the small surface area of the needle electrode and the large area of the ground pads causes the generated heat to be focused and concentrated around the needle electrode. Several electrode types are available for clinical RFA, including internally cooled electrodes and multi-tined expandable electrodes with or without perfusion [13]. An important factor that affects the success of RFA is the ability to ablate all viable tumor tissue and possibly an adequate tumor-free margin. Ideally, a 360 °, 0.5–1 cm-thick ablative margin should be produced around the tumor. This cuff would ensure that the peripheral portion of the tumor as well as any microscopic invasions located in its close proximity have been eradicated [13] (Fig. 1).
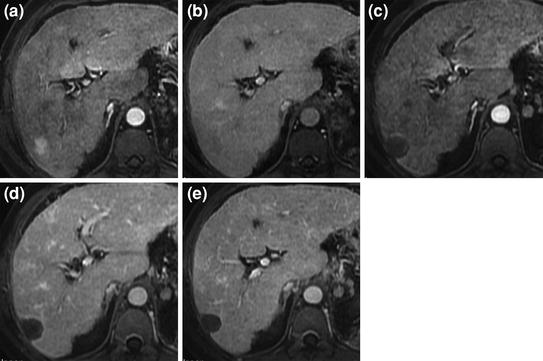

Fig. 1
Complete response of HCC after RFA. Pretreatment MRI shows small hypervascular HCC with typical arterial phase enhancement (a) and portal venous phase washout (b). MRI obtained 1 month after treatment shows larger unenhancing area in both the arterial (c) and the portal venous phase (d) replacing the tumor and consistent with complete ablation. Follow-up MRI at 6 months (e) shows sustained complete response
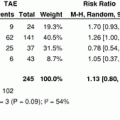
RFA has been the most widely assessed alternative to PEI for local ablation of HCC. Five randomized controlled trials (RCTs) have compared RFA versus PEI for the treatment of early-stage HCC. These investigations consistently showed that RFA has higher anticancer effect than PEI, leading to a better local control of the disease [14–18] (Table 2). The assessment of the impact of RFA on survival has been more controversial. While a survival benefit was identified in the three RCTs performed in Asia, the two European RCTs failed to show statistically significant differences in overall survival between patients who received RFA and those treated with PEI, despite the trend favoring RFA (Table 2). In patients with early-stage HCC treated with percutaneous ablation, long-term survival is influenced by multiple different interventions, given that about than 80 % of the patients will develop recurrent intrahepatic HCC nodules within 5 years of the initial treatment and will received additional therapies [19]. Nevertheless, three independent meta-analyses including all RCTs, have confirmed that treatment with RFA offers a survival benefit as compared with PEI, particularly for tumors larger than 2 cm, thus establishing RFA as the standard percutaneous technique [20–22]. For studies that reported major complications; however, the incidence in RFA-treated patients was 4.1 % (95 % CI, 1.8–6.4 %), compared to 2.7 % (95 % CI, 0.4–5.1 %) observed in PEI-treated patients [23]. This difference was not statistically significant; nevertheless, this safety profile should be taken into consideration as part of the overall risk/benefit profile in each individual case.
Table 2
Randomized controlled trials comparing radiofrequency ablation versus ethanol injection for the treatment of early-stage hepatocellular carcinoma
References | Initial CR(%) | Treatment failure *(%) | Overall survival (%) | ||
|---|---|---|---|---|---|
1-yr | 3-yr | P | |||
[14] | |||||
RFA (n = 52) | 91 | 8 | 88 | 81 | NS |
PEI (n = 50) | 82 | 34 | 96 | 73 | |
[15] | |||||
RFA (n = 52) | 96 | 17 | 82 | 74 | 0.014 |
PEI (n = 52) | 88 | 45 | 61 | 50 | |
[16] | |||||
RFA (n = 118) | 100 | 2 | 90 | 80 | 0.02 |
PEI (n = 114) | 100 | 11 | 82 | 63 | |
[17] | |||||
RFA (n = 62) | 97 | 16 | 88 | 74 | 0.031 |
PEI (n = 62) | 89 | 42 | 96 | 51 | |
[18] | |||||
RFA (n = 70) | 96 | 34 | 88 | 59 | NS |
PEI (n = 69) | 66 | 64 | 96 | 57 | |
Recent reports on long-term outcomes of RFA-treated patients have shown that in patients with Child-Pugh class A and early-stage HCC, 5 year survival rates are as high as 51–64 %, and may reach 76 % in patients who meet the BCLC criteria for surgical resection [19, 24–26] (Table 3). Caution, however, is needed when interpreting and generalizing these results, in particular in the light of studies that suggest a nonnegligible rate of incomplete histopathological response after RFA. In fact, the ability of RFA to achieve a complete tumour eradication appears to be dependent on tumor size and location. In particular, histological studies performed in liver specimens of patients who underwent RFA as bridge treatment to transplantation showed that the presence of large (3 mm or more) abutting vessels result in a drop of the rate of complete tumor necrosis to less than 50 %, because of the heat loss due to perfusion-mediated tissue cooling within the area to be ablated [27] (Fig. 2). Other clinical experiences have suggested that treatment of HCC tumors in subcapsular location or adjacent to the gallbladder is associated with an increased risk of incomplete ablation and local tumor progression [28, 29]. Treatment of tumors in such unfavorable locations has also been shown to result in a significant increase of major complications [30, 31].
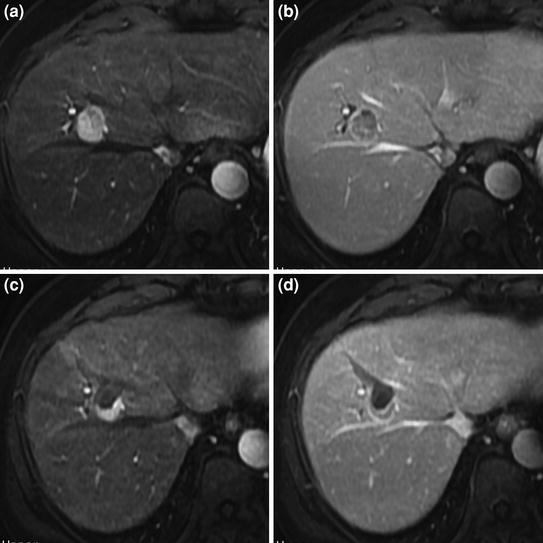
Table 3
Studies reporting 5 year survival of patients with early-stage hepatocellular carcinoma who received radiofrequency ablation as first-line nonsurgical treatment
References | Patients No | Overall survival (%) | ||
|---|---|---|---|---|
1-yr | 3-yr | 5-yr | ||
[19] | ||||
Child-Pugh A | 144 | 100 | 76 | 51 |
Child-Pugh B | 43 | 89 | 46 | 31 |
[24] | ||||
Child-Pugh A | 221 | 96 | 83 | 63 |
Child-Pugh B–C a | 98 | 90 | 65 | 31 |
[25] | ||||
Child-Pugh A | 359 | NA | 78 | 64 |
Child-Pugh B | 160 | NA | 49 | 38 |
[26] | ||||
BCLC resectableb | 67 | NA | 82 | 76 |
BCLC unresectable | 168 | NA | 49 | 27 |

Fig. 2
Incomplete response of perivascular HCC after RFA. Pretreatment MRI shows small hypervascular HCC with typical arterial phase enhancement (a) and portal venous phase washout (b), adjacent to the right hepatic vein. MRI obtained 1 month after treatment shows persistent enhacing viable tumor tissue (arterial phase, c; portal venous phase, d) in the posterior aspect of the treated lesion, contiguous to the right hepatic vein
To increase the efficacy of RFA, especially in tumors of intermediate size (3–7 cm), several authors have suggested the combined use of transcatheter arterial chemoembolization (TACE) and RFA. A combination including TACE followed by RFA has been used to minimize heat loss due to perfusion-mediated tissue cooling and increase the local therapeutic effect of RFA [32–35]. On the other hand, TACE with drug-eluting beads has been performed after an RFA procedure to increase tumor necrosis by exposing to high drug concentration the peripheral part of the tumor, where only sublethal temperatures may be achieved in a standard RFA treatment [36]. Other investigators have suggested the combined use of percutaneous approaches, such ethanol injection and RFA [37]. Unfortunately, despite several investigations reporting promising results have been reported, no definitive proof of clinical efficacy was reached, as no robust RCT comparing the survival outcomes achieved with such combinations of interventional techniques over those obtained with either therapy alone has been completed so far.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree