19 Abdomen
Computed Tomography Technology and Terminology
Three distinct generations of CT technology are in current use. In order of historical development and scanner speed, these are conventional CT, spiral (or helical) CT, and multislice (or multidetector) CT. Rapid scanning is a desirable feature in abdominal CT for two reasons. First, unlike the brain, the abdomen moves with respiration, and imaging during a single breath-hold removes the risk of respiratory misregistration (i.e., structures missed between slices because successive breath-holds are not identical). Second, many applications in abdominal imaging are affected by the time images are acquired relative to the bolus of intravenous contrast medium, and accurate diagnosis may require imaging precisely timed to one or more phases of enhancement. Godfrey Hounsfield invented CT in 1972, with initial clinical installations in 1974. The first CT scanner developed by Hounsfield in his laboratory at Electrical and Musical Industries required several hours to acquire the data for a single slice, and took several days to reconstruct the corresponding image. Data acquisition and image reconstruction became progressively faster during the 1970s and 1980s, although the speed of the scanners remained limited by the need for “stop-start,” slice-by-slice acquisition. That is, in conventional CT, an axial slice is generated by rotating an x-ray tube and detector array in a 360° circle around the patient. After a 360° rotation, the rotating gantry reverses direction to prevent disruption of the tethered cables that transfer the data from the detector array to the computer. Such sequential slice acquisition limits the speed of conventional CT, prevents volumetric data acquisition, results in slice misregistration, and limits temporal resolution such that multiphase volumetric scanning is not possible. The development of spiral (or helical) CT in the late 1980s represented a technologic breakthrough. In spiral CT, data is carried from the rotating gantry to the computer by slip rings, which allow continuous gantry rotation and data transfer. Scanning can be performed while the patient is moved slowly but continuously through the gantry. The ability to continuously scan allows for “non-stop” volumetric data acquisition. Data is gathered on a three-dimensional volume in a spiral fashion. Images are reconstructed from the data volume. Physical and clinical performance studies of spiral CT were first reported at the 1989 Radiological Society of North America meeting.1 The first commercially available spiral CT scanner was released in 1990.2
Spiral CT was an important technologic advance, but many limitations and compromises remained. The rotational speed of the gantry was generally one rotation per second, and clinical results suggested that a pitch (ratio of longitudinal distance moved by the tabletop during one gantry rotation to beam collimation) greater than 2 was undesirable. As a result, either large volumes could be covered, or thin sections acquired, but not both. The Elscint CT Twin scanner employed a dual array of two side-by-side detector rows, and represented an early version of multislice technology.3 However, although this scanner was an improvement over single-detector spiral CT in terms of coverage,4 substantial limitations remained. It was not until 1998 that a scanner with four detector rows became commercially available. Modern multislice CT using four or more detector rows essentially abolishes the remaining obstacles to rapid isotropic volumetric imaging by using multiple side-by-side detectors simultaneously. During recent years, top-of-the-line scanners have used 64 detector rows, although machines with 256 rows or flat-panel detectors are being investigated and will likely become more widely available in the next few years. Such multislice CT systems can collect multiple slices of data in approximately 300 milliseconds and reconstruct a 512 × 512 matrix image from millions of data points in less than a second. An entire body cavity (brain, chest, or abdomen) can be scanned in 5 to 10 seconds using the most advanced multislice CT systems. Faster imaging with modern scanners is due not only to multiple detector rows, but also to increased gantry rotational speed. Many commercial scanners are now capable of a complete gantry rotation in 0.3 seconds or less. Multislice CT has been a major advance in several areas of abdominal imaging, particularly those requiring imaging in multiple phases of enhancement after intravenous contrast medium administration (Fig. 19-1) and rapid near-isotropic (i.e., equal spatial resolution in all three planes) volumetric acquisition, such as CT angiography (Fig. 19-2). The ability to acquire scans of the abdomen during multiple phases of enhancement is particularly helpful when both arteriographic and parenchymal phases of enhancement are required for complete evaluation. For example, the arteriographic phase is critical for the assessment of arterial encasement in pancreatic cancer, but parenchymal enhancement phases are required for demonstration of the primary tumor and detection of hepatic metastases.
One of the unfortunate consequences of the increased ability to obtain multiphase imaging has been a corresponding increase in confusion with respect to the proper terminology for these studies.5 It has reasonably been suggested that the term phase should not be applied to noncontrast images.6 It also seems reasonable to use the term arteriographic phase for early arterial images in which contrast is primarily within the arteries, and the term parenchymal arterial phase for the subsequent period of early tissue enhancement when hypervascular masses are most prominent. Later periods of image acquisition can be referred to as portal venous or delayed, as appropriate. Until a universal terminology is established, it is probably best to avoid unclear terms such as dual or triple phase studies, and instead describe the specific phases acquired.
Cholangiocarcinoma
Pathologic Conditions
Cholangiocarcinomas are adenocarcinomas of the biliary ductal epithelium, and account for more than 90% of bile duct malignancies. Based on location, cholangiocarcinomas are divided into intrahepatic, proximal extrahepatic (i.e., perihilar), and distal extrahepatic. Perihilar cholangiocarcinomas, originating at the confluence of the right and left hepatic ducts, are the most common, and are also known as Klatskin tumors.7 Distal extrahepatic cholangiocarcinomas, originating from the common duct between the upper border of the pancreas and the ampulla of Vater, are the second most common. Intrahepatic cholangiocarcinomas are the least common. Cholangiocarcinomas are infiltrative tumors, and typically invade and occlude adjacent structures such as the bile ducts, portal veins, and hepatic arteries. Cholangiocarcinomas are often locally advanced before they present with clinical features of biliary obstruction such as painless jaundice, pruritus, dark urine, and pale stools. Regional extension occurs into the liver and the lymph nodes of the celiac and pancreaticoduodenal chains. Distant metastases may appear in the peritoneum, thoracic lymph nodes, and surgical or drain incisions. Intrahepatic cholangiocarcinomas may involve hepatoduodenal ligament, para-aortic, retro pancreatic, or common hepatic artery nodes. In addition, perihilar and left intrahepatic cholangiocarcinomas may spread along the left gastric nodes adjacent to the lesser curvature of the stomach.8
Imaging of Cholangiocarcinoma
The traditional standard of reference for the diagnosis and staging of cholangiocarcinoma has been the combination of conventional cholangiography and angiography.9 Modern cross-sectional imaging has largely replaced the need for these invasive tests, both with respect to diagnosis and staging. The diagnosis of cholangiocarcinoma should be suspected when US, CT, or MRI demonstrate dilated intrahepatic ducts with nondilated extrahepatic ducts in a patient with painless jaundice. Because of the infiltrative nature of cholangiocarcinoma, the primary tumor may or may not be seen, and visualization of a mass is not required to suggest the diagnosis. Potential differential diagnoses in this setting include postsurgical stricture (either iatrogenic bile duct injury or biliary enteric anastomotic stricture) or malignant masquerade (other hepatic masses, such as metastases, very rarely result in biliary dilatation). Postsurgical strictures are usually evident from the clinical history. Malignant masquerade is a rare nonneoplastic fibrous proliferation that occurs at the hilum of the liver and clinically and radiologically mimics perihilar cholangiocarcinoma.10 In a series of 94 patients with suspected obstructive malignancy at the hilum, 8 were found to have malignant masquerade at pathologic analysis.10 The 8 patients ranged in age from 37 to 66 years, and all presented with obstructive jaundice. One later developed cholangiographic changes of sclerosing cholangitis, and it may be that malignant masquerade represents a localized, masslike form of sclerosing cholangitis.11
Conventional Cholangiography
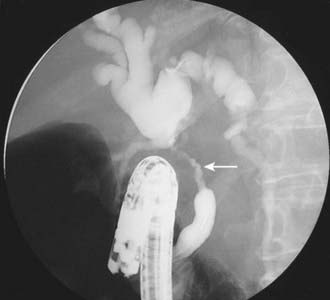
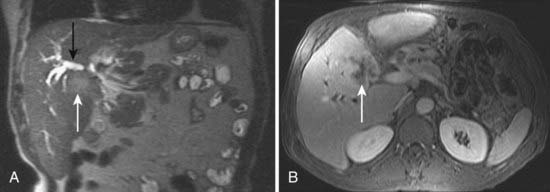
Conventional cholangiography can be performed by endoscopic retrograde cholangiopancreatography (ERCP) or percutaneous transhepatic cholangiography (PTC). ERCP has the advantage of excluding ampullary pathologic conditions by direct endoscopic evaluation and allowing for simultaneous tissue sampling. Palliative endobiliary stenting can also be performed at the same time. ERCP is usually favored more than PTC, but the latter may be appropriate when attempts at ERCP have been unsuccessful. PTC may allow access to proximal lesions with obstruction of both right and left hepatic ducts. Tissue may be obtained for cytologic analysis and drainage performed. The typical appearance of a cholangiocarcinoma at conventional cholangiography is of a tight, irregular stricture of variable length associated with marked dilatation of the proximal biliary tract (Fig. 19-3).
Ultrasound
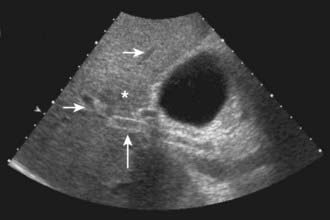
US is frequently the initial investigation requested in patients with suspected biliary obstruction. Klatskin tumors classically manifest at US as segmental dilatation and nonunion of the right and left hepatic ducts in the porta hepatis (Fig. 19-4). The papillary and nodular ductal variants of cholangiocarcinoma are relatively easy to see at US. Papillary tumors form polypoid intraluminal masses, whereas nodular cholangiocarcinoma manifests as a discrete smooth mass with associated mural thickening.12 Smaller or infiltrative tumors may be missed, although experienced operators can demonstrate high sensitivity. In a retrospective study of 49 patients with pathologically proved cholangiocarcinoma, US demonstrated the tumor in 47 (96%) cases; a mass was demonstrated in 44 and focal or diffuse thickening of the bile duct wall in 3.13 In a study of 39 patients with Klatskin tumors undergoing preoperative US,14 ductal masses were detected in 34 patients (87%). Masses were isoechoic in 22 (65%), hypoechoic in 7 (21%), and hyperechoic in 5 (15%). Morphologic tumor patterns included nodular mural thickening (n = 19; 56%), irregular infiltration (n = 9; 26%), and intraductal polypoid masses (n = 6; 18%). Vascular involvement is an important determinant of irresectability, and portal vein involvement can be assessed with an accuracy of up to 91% by Doppler US.15 In a study of 22 patients with hilar cholangiocarcinoma, vascular involvement was evaluated by Doppler US and arteriography, using operative findings as the standard of reference. Vascular patency or involvement was correctly determined by Doppler US in 19 patients (86%), compared with 18 by arteriography (82%).16 EUS enables both bile duct visualization and nodal evaluation, and EUS-guided fine-needle aspiration biopsy may be diagnostic when other diagnostic tests are inconclusive.17
Computed Tomography
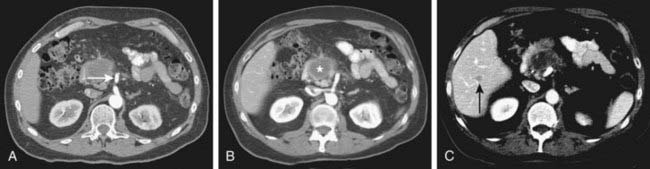
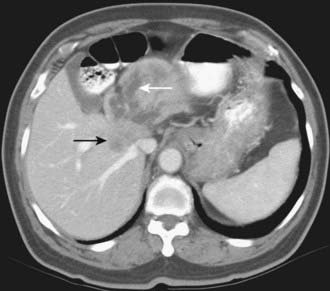
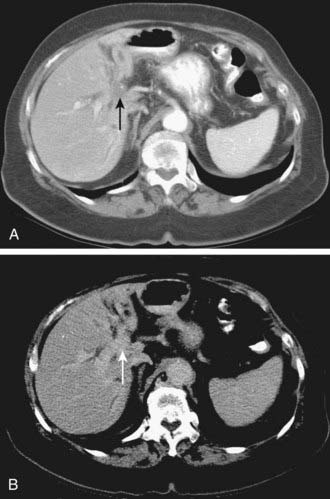
Depending on referral practices, CT may be the first investigation requested in patients with painless jaundice. The CT findings in Klatskin tumor resemble those seen at US, namely segmental dilatation and nonunion of the right and left hepatic ducts in the porta hepatis (Fig. 19-5). The reported frequency with which a primary tumor mass is seen varies from 40% to 91%.18–21 Delayed enhancement is a relatively recently described finding that has been reported in 36% to 74% of cases (Fig. 19-6).20,21 In one study, 3 of 47 cholangiocarcinomas were seen only in delayed images.21 The optimal time for acquisition of delayed images is 10 to 20 minutes after contrast media injection.20 Lobar atrophy is a nonspecific but useful ancillary feature of cholangiocarcinoma that may be better appreciated at CT than US. Lobar atrophy usually indicates both biliary and portal venous obstruction in that lobe. Another advantage of CT is the ability to detect metastases, such as nodal or peritoneal deposits. Adenopathy in cholangiocarcinoma arising secondary to primary sclerosing cholangitis should be interpreted with caution, because nodal enlargement in this context may be reactive or metastatic. Occasionally, CT may suggest the diagnosis of peripheral intrahepatic cholangiocarcinoma when a patient with vague or nonspecific symptoms is found to have a hypodense hepatic mass with peripheral enhancement, biliary dilatation, and delayed enhancement.22
Magnetic Resonance Imaging
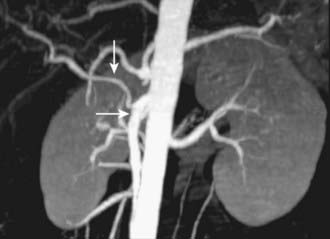
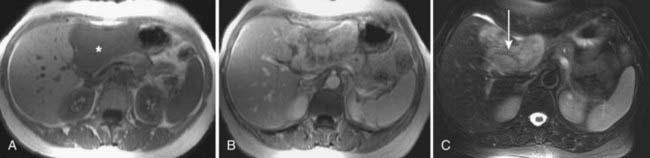
MRI findings in cholangiocarcinoma resemble those seen at CT: isolated intrahepatic biliary dilatation with or without a visible mass (Fig. 19-7). Although MRI is frequently reserved for patients with contraindications to contrast-enhanced CT, such as elevated creatinine or contrast allergy, the modality has several attractive imaging characteristics that may assist in tumor evaluation. MRI cholangiography provides similar information to conventional cholangiography, and may obviate the need for invasive biliary imaging.23 MRI angiography can be used to assess vascular involvement and may replace catheter angiography for vascular evaluation. In a study of 27 preoperative patients with Klatskin tumors, evaluation of vascular involvement by MRI and US were compared with intraoperative findings.24 Encasement, thrombosis, occlusion, and nonvisualization were considered to be evidence of vascular involvement. Of 16 involved veins, 12 were detected at MRI compared with 13 of 16 at US. There was one false-positive diagnosis of inferior vena cava involvement at both MRI and US. These results suggest that MRI and US provide comparable results for assessment of hepatic vein involvement by tumor. Central hypointensity on T2-weighted images appears to be a characteristic finding in intrahepatic cholangiocarcinoma (Fig. 19-8), and reflects severe fibrosis.25
Positron Emission Tomography
Early results suggest PET scanning may have a role to play in the evaluation of cholangiocarcinoma,26 because it may detect cholangiocarcinoma tumors as small as 1 cm in diameter and can simultaneously detect distant metastases. In a study of 54 patients, 26 with cholangiocarcinoma and 28 with no disease or benign disease, true-positive PET scans were obtained in 24 of 26 cholangiocarcinoma patients with only two false-positive results (sensitivity of 92% and specificity of 93%). Regional or hepatoduodenal lymph node metastases were detected with PET in only 2 of 15 cases, whereas distant metastases (peritoneal carcinomatosis, pulmonary metastases) were diagnosed in 7 of 10 cases.
Gallbladder Carcinoma
Imaging of Gallbladder Carcinoma
GBC is usually advanced at the time of diagnosis, and early-stage tumors are rarely encountered.27 As a result, imaging findings are usually relatively obvious, and can be well assessed by US, CT, or MRI. Radiologic findings include focal or diffuse thickening of the gallbladder wall (49%), a solid mass replacing the gallbladder in the gallbladder fossa (37%), or an intraluminal mass in the gallbladder (14%). Associated findings include invasion of adjacent structures such as the liver (67%), coexistent gallstones (64%), biliary duct dilatation (38%), coexistent porcelain gallbladder (4%), and distant metastases outside the liver (3%).28 Although gallbladder cancer may manifest as an intraluminal mass at imaging, it should be remembered that gallbladder polyps may be found in up to 2.5% of asymptomatic patients29 and that most small, noncalculous gallbladder filling defects are benign. In a study of 143 gallbladder polyps less than 1.5 cm in diameter removed by laparoscopic cholecystectomy, the final diagnoses were cholesterol polyp (74%), adenoma (11%), adenomyomatosis (7%), adenocarcinoma (4%), and hyperplastic or inflammatory polyp (4%).30 Historical reports have suggested the risk of GBC in patients with a porcelain gallbladder is between 12% and 61%,31 and such numbers are frequently quoted in clinical practice. Two recent series of 10,741 and 25,900 cholecystectomy specimens from the University of California, Los Angeles, and Massachusetts General Hospital, respectively, have suggested the true risk is 0% to 7%.31,32