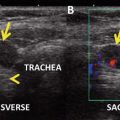
Fig. 27.1
Posttreatment 131I SPECT-CT reveals intense uptake in a left retropharyngeal LN (node of Rouviere) and to a lesser extent in a right retropharyngeal LN
Assessment and Literature Review
Thyroid cancer is rare in the pediatric population , but the incidence appears to be increasing, especially in adolescents. PTC is the most common variant and accounts for 90 % or more of childhood thyroid cancers. Pediatric PTC is frequently multifocal and bilateral. Regional lymph node metastases occur in >80 % of cases and are often the reason a child with PTC comes to clinical attention [9, 10]. Children with a significant cervical disease burden are at the highest risk of hematogenous metastases to the lungs, which occur in up to 25 % of cases [5, 9, 11–13]. Despite the apparent aggressive clinical presentation of PTC when diagnosed during childhood and a higher rate of pulmonary metastases compared with adults, disease-specific mortality in pediatric PTC is extremely low (~2 % or less decades after diagnosis) [5, 7, 8, 14, 15]. This excellent long-term prognosis, coupled with unique concerns regarding the potential late sequelae related to overzealous treatment at a young age (e.g., second malignancies [4, 6–8] and pulmonary fibrosis [16]), makes the management of pediatric PTC with lung metastases challenging. Furthermore, there have been no prospective clinical trials to guide decision-making, and our understanding of pediatric PTC primarily comes from adult PTC, in addition to clinical reviews, pediatric PTC case series, and expert opinion. It is only recently that formal guidelines for the management of pediatric PTC have been developed by the American Thyroid Association (ATA) [1].
In most cases, it is recommended that the initial evaluation of children with PTC includes a preoperative CXR to assess for macroscopic lung metastases; the finding of which may alter plans for subsequent RAI therapy [2]. However, a CXR is not sensitive enough to identify small-volume micronodular pulmonary metastases [13], and for that reason, some centers also consider chest CT scanning at diagnosis, especially in children with bulky cervical disease, who are at the highest risk for distant metastases [11]. Current pediatric guidelines do not advocate routine chest CT in all patients [1], with the additional understanding that postoperative staging with RAI, if indicated, will effectively identify most children with pulmonary metastases, even those with negative baseline radiographic imaging [13]. In the end, knowledge regarding the presence or absence of pulmonary metastases does not alter the plan for initial therapy, which is the appropriate oncologic surgery (total thyroidectomy ± compartment-oriented neck dissection) by a high-volume thyroid surgeon.
The inaugural pediatric ATA guidelines introduced a postoperative risk categorization (ATA pediatric low, intermediate, and high risk) based upon TNM staging that helps to identify patients at risk of distant metastases and thereby determine which children should undergo routine postoperative staging with RAI [1]. Using this novel classification, children with significant central or lateral neck lymph node involvement are considered intermediate or high risk, depending on the extent and volume of disease. In most intermediate- (clinical N1a or microscopic N1b disease) and in all high-risk (clinical N1b disease) patients, including patients already known to have distant metastases, postoperative staging with a diagnostic RAI scan and a TSH-stimulated Tg is recommended [1, 2].
In some children, the diagnostic whole-body scan may be negative for lung uptake (Fig. 27.2a), but the Tg may be significantly elevated, thereby suggesting the presence of distant metastases. The absolute value of hyper-thyroglobulinemia that predicts lung metastases in children remains largely unstudied, but empiric RAI therapy is usually administered to a child who is high risk for distant metastases (based upon the ATA Pediatric risk categories) and whose stimulated Tg is >10 ng/ml [1]. Although the empiric use of 131I is not associated with cure in adults with negative diagnostic RAI scans and known structural disease [17], this issue remains unstudied in children, who may be more prone to benefit from RAI in this setting due to the inherent differences in tumor biology. In all cases, after 131I therapy, a post-therapy scan is recommended 4–7 days in order to identify iodine-avid disease that may not have been readily visible on the diagnostic whole-body scan and also to help determine iodine avidity in known or suspected metastatic disease previously seen on radiographic imaging (Fig. 27.2b).
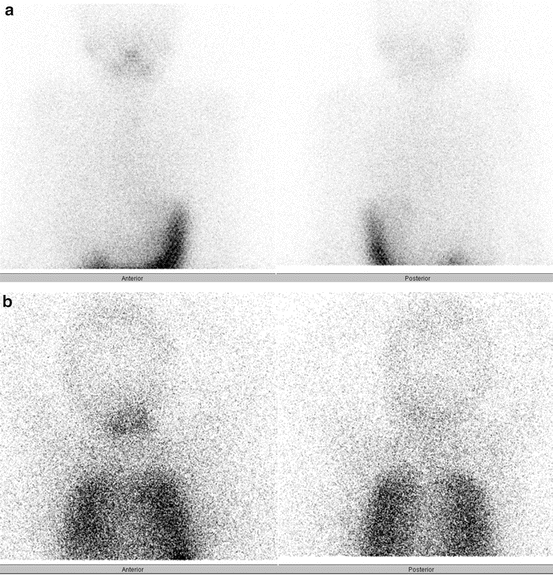

Fig. 27.2
Diagnostic (a) and posttreatment (b) 131I scans in a 12-year-old boy with stage II PTC. The diagnostic study revealed no evidence of iodine-avid pulmonary metastases, but he was treated empirically with 131I due to a stimulated Tg of 767 ng/ml and known pulmonary metastases that previously demonstrated iodine avidity. The posttreatment scan 7 days after 131I clearly demonstrates diffuse pulmonary metastases
The goal of 131I therapy in children with pulmonary metastases is to decrease the risk of thyroid cancer progression and theoretically to improve mortality by eliminating iodine-avid disease while reducing the risk of late complications, such as pulmonary fibrosis [16]. The intent of therapy is no longer to render every patient with a negative thyroglobulin, since that is achievable in only about 50 % of pediatric patients with pulmonary metastases [1, 5].
Those children identified to have iodine-avid distant disease will likely benefit from 131I therapy. Therefore, 131I is typically administered at an empiric activity that is proportionately equivalent to a 150–200 mCi dose in an adult (sometimes less, if significant diffuse pulmonary uptake is present). Dosimetry is considered in children with diffuse pulmonary metastases but is not readily available in all centers. In these cases, the goal is to ensure that the whole-body retention 48 h after 131I administration does not exceed 2.96 GBq (80 mCi) in the presence of iodine-avid diffuse lung metastases [18], which should minimize the very real concern of pulmonary fibrosis in this population [16].
Children with small-volume, iodine-avid micronodular (<1 cm) lung disease are the most likely to respond to treatment [19], and they may ultimately become disease-free, whereas others, especially those with a more extensive metastatic disease burden, may never become cancer-free as assessed by Tg levels [3–5, 19]. The nadir response to RAI can take 1–2 years or more [3], and because of this, current recommendations are to wait until the disease either progresses or unequivocally remains persistent before proceeding to subsequent evaluation and treatment with 131I [1, 2]. The thought is that such a cautious approach to repeated 131I therapy may help to mitigate long-term sequelae of RAI, while also ensuring durable control of disease. Further studies are required regarding the optimal dosing and timing of 131I for children with PTC and iodine-avid pulmonary metastases. In all cases, the decision to treat a child with RAI should be individualized [1, 2], preferably by clinicians experienced in the management of advanced pediatric PTC.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree