Fig. 34.1
18Fluorodeoxyglucose positron emission tomography (18FDG PET)/CT scan performed at initial staging. (a) 18FDG PET/CT whole-body maximum intensity projection (MIP) showing uptake in 6 cm left lobe thyroid cancer (SUVmax: 6) and in the third left rib (SUVmax: 3.4) corresponding to a 4 cm lytic lesion. There was no significant FDG uptake in multiple lung nodules of few millimeters diameter. (b) Axial fusion image showing high FDG uptake in the third left rib lesion. (c) Axial fusion image showing no significant FDG uptake in the right ischial lesion
A total thyroidectomy and central neck dissection were performed. Final histology showed a polymorphic thyroid cancer measuring 10 cm with poorly differentiated areas, 6 mitoses × 2 mm2, Ki 67 15 %, and several foci of necrosis. Due to massive extrathyroidal extension, the tumor was classified as pT4. No metastatic lymph nodes were found (N0). Two months after surgery, the patient received 100 mCi (3.7 GBq) of radioactive iodine (131I) after thyroid hormone withdrawal for 4 weeks. Thyroglobulin level was 3726 ng/ml with a TSH level of 35 mIU/l. Post-therapy whole-body (WB) scan showed high 131I uptake in the thyroid remnants and diffuse uptake not only in the lung and in the rib lesion but also in T8 and right iliac bone lesions. Furthermore, 131I single-photon emission tomography (SPECT)/CT scan detected three other bone lesions in the spine (C4 and L1) and in the pelvis (left iliac bone), respectively, not visible on WB scan (Fig. 34.2a, b). On the CT component, the rib and the ischial lesions both appeared as lytic lesions of 4 cm and 2.3 cm, respectively, while there was no evidence of bone lesions in the other bone 131I foci. In particular, the ischial lesion showed cortical lysis with risk of fracture (Fig. 34.2c–e). Cryoablation for the rib lesion and cryoablation plus cementoplasty for the ischial lesion were performed. During local treatment, a biopsy of the ischial lesion was performed and was consistent with well-differentiated thyroid cancer.
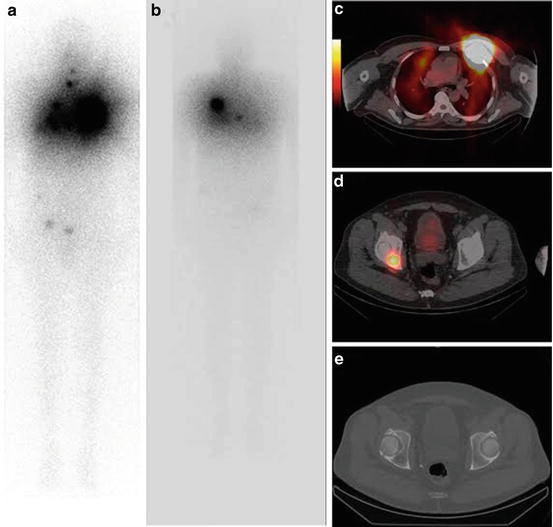

Fig. 34.2
131I post-therapy whole-body (WB) scan after administration of 100 mCi (3.7 GBq) after thyroid hormone withdrawal for 4 weeks. (a, b) Anterior and posterior view showing high 131I uptake in the third left rib lesion, in the right ischial lesion, and in the spine T8 and a diffuse uptake in the lung. (c) Axial fusion image (SPECT/CT) showing high 131I uptake in the third left rib. (d) Axial fusion image (SPECT/CT) showing high 131I uptake in the right ischium. (e) Axial image of CT component of SPECT/CT showing a 23 mm lytic lesion in the right ischium
Assessment and Literature Review
Bone metastases occur in less than 5 % of patients with thyroid cancer and are often a cause of morbidity from local pain, fracture, and neurological complications [1]. The median reported overall survival in case of bone metastases is 2–4 years after their diagnosis , but may range from 96 % at 10 years in young patients (<45 years) without radiological abnormalities to less than 10 % in older patients with multiple lesions and radiological abnormalities [1–3]. In the presence of 131I uptake in the metastases, 131I is used as first-line treatment [4]. Although 131I may eradicate small metastases, it is poorly effective at treating large metastases, and in such cases, local treatment modalities are also considered. In patients with radioactive iodine refractory thyroid cancer, systemic treatments such as tyrosine-kinase inhibitors or chemotherapy are less effective in bone lesions than in treating visceral metastases, and again, local treatments are considered [4, 5]. Data on the management of bone metastases from thyroid cancer are rare and focused on surgery or radiotherapy [6–9]. Minimally invasive thermal ablation techniques show promising results in terms of pain control, efficacy in local tumor control, and lesion stabilization in case of fracture risk in patients with bone metastases from non-thyroid cancers [10–13]. Only a few cases of thyroid cancer treated by thermal ablation have been reported, and additional studies are necessary to determine the role of thermal ablation in patients with bone metastases from thyroid cancer [14–16].
Characteristics of Bone Lesions from Differentiated Thyroid Cancer
Thyroid cancer bone lesions are often lytic and can be associated with extension into surrounding soft tissues [17]. The spine is the most frequent site of bone metastases, and spinal metastases can be the first manifestation of follicular thyroid cancer [17, 18]. The major risk is cortical rupture and local complications such as medullary compression or fracture [18]. Bone metastases are often vascular and this pattern makes them accessible to embolization. In patients with bone metastases, one of the most important prognostic factors is the extent of the disease (size and number of lesions) [1, 2]. Whole-body functional imaging can give important information on the patient’s stage and prognosis. In particular, both FDG PET/CT and WB 131I post-therapy scans are recommended to stage metastatic patients at the time of diagnosis, as they will provide important information for both prognosis and therapy. Bone lesions are frequently FDG avid due to their vascularity and concomitant inflammatory bone reaction. High FDG uptake in distant metastases is a negative prognostic parameter and a negative predictive factor for response to radioactive iodine [19–22]. However in most differentiated tumors, WB 131I post-therapy scan can show radioactive iodine uptake in small lesions that are not visualized on cross-sectional imaging. In addition to 131I scans and FDG/PET CT, magnetic resonance imaging (MRI) is useful to evaluate the presence of local complications and medullary compression, which can help to plan radiotherapy.
Treating Bone Metastases with Radioactive Iodine
Radioactive iodine administration is recommended to explore 131I avidity of distant metastases, to stage the disease, and to treat 131I-avid distant metastases [4]. Two thirds of patients with distant metastases show radioactive iodine uptake, and complete response is achieved in around 40 % of these cases with initial RAI therapy, especially in case of young patients with 131I-avid micrometastases without correlates on cross-sectional imaging [1]. The prognostic value of radioactive iodine uptake in patients with bone metastases has been reported [7]. Young patients with small or single bone lesions or with bone 131I uptake but with no lesion visualized on CT can be treated by repeated 131I administrations [2, 23]. The number of 131I treatments and administered activity varies depending on institutional protocols. On the other hand, 131I is not sufficient for large or multiple bone lesions and local treatment may be warranted.
In patients with distant metastases, 18FDG PET/CT has to be correlated with 131I treatment, especially at initial staging, to evaluate FDG uptake in distant lesions and to predict response to therapy. Patients with FDG-avid and no 131I-avid distant metastases have rapidly progressive disease. In contrast, patients with 131I-avid and FDG-negative lesions have a much better prognosis. Patients with both FDG and 131I uptake in the same lesions or FDG and 131I uptake in different lesions represent a very heterogeneous group, but their prognosis seems similar to the group with only FDG uptake.
Indications and Options of Local Treatment
In case of symptomatic lesions, fracture, local compression, or spinal neurological damage, surgery is the first therapeutic choice [8]. If complete and curative surgery can be achieved, this may improve patient survival, especially in young patients [7–9]. External beam radiation therapy has been used in association with surgery for pain palliation, but stereotactic radiotherapy is becoming a very promising technique in spinal tumors as a selective and curative treatment of bone metastases [6, 24]. Some cases of vascular embolization by polyvinyl alcohol particles have also been reported with effective and immediate pain relief after treatment [25]. Vascular embolization is routinely performed before surgery to limit bleeding. In cases of limited bone lesions without soft tissue involvement but symptomatic or at risk of fracture, local thermal ablation by radiofrequency ablation (RFA) or cryotherapy is currently used more and more frequently. These techniques are preferred to other local treatment modalities because they are well tolerated, they are minimally invasive, and they can be repeated in the same patient together with cementoplasty for lesion stabilization [13]. All local treatments can be used in association with systemic treatments, such as radioactive iodine, if the lesions are 131I avid or TKIs in the case of radioactive iodine refractory cancer.
Principles and Definition of Thermal Ablation
The principle of thermal ablation action is coagulative tissue necrosis by heating a tumor with high temperature (radiofrequency ablation) or freezing it with pressurized gas (cryoablation) [26, 27]. The main effects are intracellular, vascular, and interstitial damage causing cell apoptosis. Radiofrequency ablation and cryoablation are currently the most frequently used percutaneous minimally invasive techniques, but other options such as high-intensity focused ultrasound (HIFU), irreversible electroporation, or laser ablation are evolving [28]. The selective action is achieved by inserting needles (RFA) or cryoprobes (cryoablation) under imaging guidance during the procedure. After thermal ablation of soft tissue lesions, progressive lesion shrinkage can be monitored with imaging (CT or MRI) with a fibrotic scar as final result. In some patients, 18FDG PET/CT can be more sensitive in the detection of persistent disease or disease relapse earlier than anatomical imaging [29]. In contrast, the response to thermal ablation in bone lesions is more difficult to evaluate because frequently there are no changes in size or volume of treated lesions on cross-sectional imaging. In patients with bone lesions at risk for fracture due to metastases in the spine or in femoral region, percutaneous cementoplasty with cement injection can be used together with thermal ablation to stabilize the bone and for an analgesic effect [30]. Finally, screws can be also inserted percutaneously in lesions with high risk of fracture to consolidate the bone.
Thermal Ablation for Treatment of Bone Metastases
Thermal ablation has been used for several years for treating benign bone tumors and for palliation of bone metastases. The first cases examining the feasibility of percutaneous ablation and cementoplasty efficacy in treating bone metastases were reported in 1995–2000 [31–33]. In particular, these techniques have been reported as safe and effective in reducing pain and in stabilizing lesion preventing skeletal events in bone metastases [31–33]. More recently, some investigators demonstrated efficacy as curative treatment in bone metastases from solid tumors other than thyroid cancer [10, 12, 13]. In particular, Deschamps et al. evaluated the rate of complete response to thermal ablation in 89 patients with 122 bone metastases from solid tumor. The 1-year complete treatment rate was 67 %. Oligometastatic status, metachronous metastases, and small lesions without cortical bone erosion or surrounding neurological structures were all predictive factors on multivariate analysis [13]. Only a few cases with bone metastases from differentiated thyroid cancer treated with thermal ablation have been reported. All cases showed good local control with improvement of patient quality of life after the procedure. In addition to being a palliative treatment for symptomatic lesions, percutaneous ablation may also be curative in patients with localized lesions, with a favorable impact on patient survival. In three patients with bone metastases from differentiated thyroid cancer treated by RFA in association with radioactive iodine, two of three patients with lesions of 30 and 50 mm were free of disease 44 and 53 months, respectively, after ablation [14]. In one case, two repeated RF ablation treatments at 12-month intervals were necessary to achieve complete tumor regression. The third patient showed disease progression 9 months after treatment. In eight patients with symptomatic spinal metastases from thyroid cancer, treatment included a surgical approach in the case of spinal compression or percutaneous vertebroplasty associated with systemic treatment (radioactive iodine or chemotherapy). The authors confirmed that local treatment can improve patients’ quality of life by reducing pain and prolonging time to skeletal events, especially spinal cord compression, and can delay initiation of systemic treatment. Finally, local treatment can improve patient survival, with a median survival reported in this paper of 50 months after treatment [16].
Further studies are needed to evaluate the efficacy and the impact of thermal ablation on prognosis. Preliminary results show that FDG PET/CT scan can be a useful tool for treatment follow-up also in bone lesions [34].
Thermal Ablation on Metastatic Sites Other Than Bone
Thermal ablation can also be performed for liver and lung metastases . Clinical trials show high efficacy of RFA on liver lesions from solid tumors, with local control equivalent to surgical resection ranging from 40 to 80 %, and a prolonged overall survival in treated patients [35, 36]. In neuroendocrine tumors, treatment of liver lesions by thermal ablation is now considered an alternative to surgery, especially when there is a small number of lesions of small diameter (< to 3 cm) [37]. A few cases of liver metastases from thyroid cancer treated by thermal ablation have been reported. In three patients with liver metastases from thyroid cancer (two medullary thyroid cancer and one follicular thyroid cancer) treated by RFA, thermal ablation reduced local symptoms due to hepatic capsular compression [38].
In a clinical trial focused on lung lesions, RFA was both effective and well tolerated, with a high complete tumor control rate (93 %) at 18 months in 100 analyzed lung lesions, including primary lung tumors and distant metastases from solid tumors [39]. Another multicenter prospective trial including 183 lung metastases showed again a high complete response rate (88 %) at 1 year and an overall survival of 92 % and 64 % at 1 year and at 2 years, respectively [40]. In all clinical trials, lesion size and lesion location are reported as the most important predictive factors of response. In particular, recurrence occurs more frequently in lesions >3 cm with soft tissue or mediastinal invasion and if the lesion is in contact with large vessels [39]. FDG PET/CT is a useful tool to evaluate response to treatment and to detect early relapse of disease when the lesions have FDG uptake on a baseline FDG PET/CT [29].
Denosumab and Bisphosphonates
To treat bone lesions, some systemic specific bone agents such as bisphosphonates and more recently the anti-RANK agent denosumab have demonstrated efficacy in reducing skeletal events in patients with bone metastases from prostate or breast cancer [41]. A beneficial effect of these agents has also been reported in patients with lytic lesions from other solid tumors such as lung, renal cell, or myeloma due to inhibition of osteoclast action. In particular, a beneficial effect of zoledronic acid treatment in terms of fewer and delayed skeletal events has been reported in some patients with bone lesions from thyroid cancer, leading to consideration of this drug as a valid therapeutic option [42]. On the other hand, no data are available on denosumab’s efficacy on bone lesions from thyroid cancer, although it is a promising and potentially more effective therapy than zoledronic acid in other tumors [43]. They both may be useful in the case of disseminated and progressive bone metastases. Bisphosphonates and denosumab are administered monthly by intravenous or subcutaneous injection, respectively, with careful follow-up to monitor for jaw osteonecrosis, hypocalcemia, and renal failure that are the most common side effects. To avoid hypocalcemia, calcium and vitamin D therapy is recommended. Bisphosphonates and denosumab are not curative therapies, but they can be used in association with local treatment for symptomatic lesions or lesions at risk or with other systemic treatments such as TKI agents .
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree