Fig. 10.1
Follicular thyroid cancer demonstrating capsular invasion of tumor
Literature Review
Definition
Follicular thyroid cancer (FTC) is a differentiated thyroid cancer, representing approximately 5.5 % of all thyroid malignancies [1]. Histologically, FTC is characterized by the absence of nuclear features of papillary thyroid carcinoma and the presence of capsular or vascular invasion; therefore, histologic rather than cytologic evaluation of tumors is mandatory to discern between malignant lesions and benign adenomas [2, 3]. The World Health Organization (WHO) histologic classification of thyroid tumors suggests a subclassification of FTC based on the degree of invasiveness, into minimally invasive follicular cancer (MIFC) and widely invasive follicular thyroid cancer (WIFC) . According to the WHO, MIFC is an encapsulated follicular tumor of low malignant potential; unequivocal invasion of the blood vessels within or immediately outside of the tumor capsule and/or invasion that penetrates the full thickness of the capsule is required for diagnosis. WIFC is characterized by widespread infiltration of blood vessels and/or surrounding parenchyma and tissues, often lacking complete encapsulation [4]. FTC also may present as an oncocytic variant, Hürthle cell FTC, which is recognized by the WHO and characterized by the presence of >75 % of metaplastic cells, along with an abnormal accumulation of altered mitochondria [3, 5].
However, the criteria for diagnosis of MIFC are still controversial, even among experienced pathologists [6–12]. Although the WHO definition of a MIFC is a follicular tumor with complete capsular invasion, some consider even a focal extension into the capsule to be diagnostic for malignancy [10, 13, 14]. This diagnosis is made more difficult by the potential for disruption of the nodule at the time of FNA, by an intraoperative disruption of the capsule during thyroidectomy, or pathologic artifacts; all of these factors may be difficult to distinguish from neoplastic capsular invasion. Hence, the integrity of the tumor capsule is fundamental to allow proper diagnosis [15].
Similarly, the definition of vascular invasion is not consistent in the literature; some investigators diagnose MIFC only when fewer than four blood vessels are involved [16, 17]. In contrast, an update to the College of American Pathologists’ thyroid cancer protocol published in 2009 reports that some authors believe that the presence of any vascular infiltration negates a diagnosis of MIFC due to the more aggressive behavior that these tumors demonstrate [8, 18]. In spite of controversies, there is consensus that histological examination of a minimum of ten tissue blocks is necessary for confirmation of FTC in encapsulated neoplasia.
Molecular Analysis
Over the past decade, research has focused on identifying potential genetic mutations in thyroid cancer that could be translated into clinical practice to complement preoperative FNA testing [19–21]. More than 70 % of differentiated thyroid cancers are found to carry identified genetic mutations, including BRAF and RAS point mutations and RET/PTC and PAX8/PPARγ gene fusions. These alterations can be detected in thyroid FNA and surgical specimens and molecular markers of diagnostic and prognostic significance. In fact, the 2009 revised American Thyroid Association (ATA) guidelines suggest that molecular markers such as BRAF, RAS, RET/PTC, and PAX8/PPARγ can be considered in the management of patients with indeterminate FNA cytology reports [22]. Specifically, PAX8/PPARγ rearrangement , which is a fusion of the PAX8 gene and the peroxisome proliferator-activated receptor (PPARγ) gene, is present in 30–40 % of conventional FTC and less often in oncocytic variants of FTC [20]. FTC patients with the mutation often present at a younger age have smaller tumors, and the tumors often have vascular invasion. While the presence of the mutation is not diagnostic of malignancy, it should prompt a more thorough search for potential capsular and vascular invasion. FTC also is associated with Cowden syndrome, which is within the spectrum of the phosphatase and tensin (PTEN)—hamartoma tumor syndrome caused by a germline mutation of the PTEN tumor suppressor gene. Up to 10 % of patients will develop thyroid cancer, most commonly FTC [23].
Recent studies have focused on other molecular analyses to identify potential markers of MIFC. Hunt et al. assessed a panel of ten tumor suppressor genes (L-MYC, CMM, hOGG1, VHL, APC, MCC, MTS1/p16, pTEN, p53, and NF2) to study loss of heterozygosity mutations, with the hypothesis that genotyping follicular-derived thyroid neoplasms may predict histologic aggressiveness [24]. Despite a limited sample size (8 follicular adenomas, 5 MIFC, and 5 WIFC), the authors found that the frequency of allelic loss (FAL) did correlate with histologic aggressiveness; follicular adenomas had a FAL of only 9 %, compared with a FAL of 30 % for MIFC and 53 % for WIFC. Lubitz et al. performed a microarray analysis of 13 follicular adenomas, seven MIFC, and seven WIFC to generate a list of differentially expressed genes and found that while many MIFCs were genetically similar, MIFC had 223 differently expressed genes, compared with follicular adenomas, and 365 differently expressed genes compared with WIFC [25]. This suggests that gene profiling may be useful in the molecular pathogenesis of MIFC and in predicting malignancy potential. Lastly, in a study of 34 patients with MIFC, including 12 with distant metastases and 13 patients with WIFC, comprehensive expression profiling was performed utilizing real-time PCR and PCR arrays to identify novel prognostic factors for metastatic MIFC using microRNA [26]. The authors found that several microRNA clusters correlated with metastatic MIFC, and that these expression levels were similar to those of patients with WIFC, suggesting a close similarity between metastatic MIFC and WIFC.
Prognosis
The subclassification between MIFC and WIFC carries a relevant prognostic meaning, given the excellent long-term prognosis and low rates of recurrent disease associated with MIFC [6, 10, 27, 28]. One of the earliest studies on the outcomes of patients with FTC was a review of 72 patients at the Mayo Clinic; 20 patients had capsular invasion only, and 45 patients had vascular invasion, with or without capsular invasion. After a median follow-up of 11 years, 10-year cause-specific mortality was 0 % for patients with capsular invasion and 28 % for patients with vascular invasion (p = 0.019); the 10-year rates of development of distant metastases were 0 % and 19 %, respectively (p = 0.052). This suggests that patients with FTC and only capsular invasion (the equivalent of the current definition of MIFC) represent a subgroup of patients that have excellent outcomes and therefore can be managed more conservatively [11).
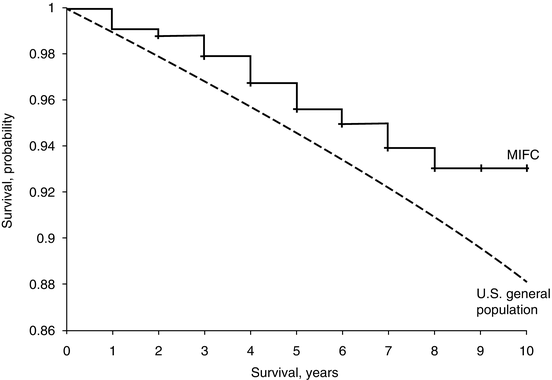
A recent retrospective study from the Surveillance, Epidemiology, and End Results (SEER) database analyzed the largest published series of MIFC; a total of 1,200 patients with MIFC and 4,208 with WIFC were identified between 2000 and 2009. MIFC tumors were less likely than WIFC tumors to involve lymph nodes (0.9 vs. 3.6 %; p < 0.001) and metastasize (0.5 vs. 8.9 %; p < 0001). At last follow-up, a significantly greater proportion of patients with MIFC were alive (96.8 vs. 86.5 % WIFC; p < 0.001). Of particular interest was that only two MIFC patients died of disease-specific causes (disease-specific survival rate, 99.8 %), compared to a disease-specific survival rate of 94.8 % in patients with WIFC (p < 0.001). The overall survival rate of patients with MIFC was found to be comparable to that of the general US population (Fig. 10.2) [29]. In contrast, in a single institutional series of 251 Japanese patients diagnosed with MIFC from 1989 to 2006, Sugino et al. found a compromised cause-specific survival of 95.2, 89.5, and 84.5 % at 10, 15, and 20 years, respectively [30]. Distant metastases were identified in 54 (22 %) patients, including 22 patients with metastases identified at the time of initial surgery. On univariate analysis, tumor size ≥4 cm and patient age ≥45 years were associated with distant metastases. An explanation for such a divergence in survival between this study and the SEER study could be secondary to the higher rate of metastatic MIFC of the Sugino study: 21.5 % compared to 0.6 % of patients in the SEER database. The reason for the different rates of metastases between these two studies is not clear but may be due to differences in the cohort of analyzed patients; the Japanese manuscript included MIFCs with a larger mean tumor size than the US population-based analysis (4.4 cm vs. 3.4 cm, respectively) as well as pediatric patients, while the SEER study was limited to adults only.
Several other factors have been reported to affect prognosis of patients with MIFC. Patient age ≥45 years at the time of diagnosis is an established predictor of compromised survival for patients with differentiated thyroid cancers; the American Joint Committee on Cancer (AJCC) staging system utilizes two different staging systems for patients with differentiated thyroid cancer [31–33]. Patients <45 years are considered to have stage I disease, irrespective of the extent of locoregional metastases, unless distant metastases are present, in which patients are considered to have stage II disease. In contrast, patients ≥45 years are divided into stage I–IV disease, and those with locoregional lymph node metastases are classified as having stage III (N1a) or stage IV (N1b) disease. Ito et al. recently analyzed a cohort of 292 patients who underwent initial surgery between 1983 and 2007 and showed that MIFC in patients ≥45 years had compromised disease-free survival and cause-specific survival compared to similar patients who were younger [34]. Interestingly, the disease-free survival of patients <20 years of age tended to be reduced compared to patients aged 20–44 years; nevertheless, none of the patients <20 years died of their disease. Whereas previous studies have failed to demonstrate that vascular invasion was a negative prognostic factor, in 2013 two sets of investigators, Kim et al. and Ito et al., showed that extensive involvement of blood vessels was associated with increased disease-specific mortality in series of 165 and 292 MIFCs, respectively [11, 13, 27, 35]. This finding might be a helpful contribution to the debate regarding the definition of pathologic criteria for MIFC. Moreover, tumor size >4.0 cm has been reported to be associated with compromised outcomes in these patients [30, 36]. This is not surprising, considering that the 4.0 cm cutoff is taken into account both in the AJCC staging system and in the ATA guidelines for the management of thyroid nodules and differentiated thyroid cancer [22, 33].
The prognosis of the Hürthle cell variant of FTC has been controversial and ranges from a low malignant potential tumor to a more aggressive one. In a review of all thyroid cancer cases captured in the National Cancer Data Base, Hundahl et al. demonstrated that patients with the Hürthle cell variant of FTC had similar 5-year survival rates to patients with FTC, but at 10 years, patients with the Hürthle cell variant had a lower overall survival rate (76 vs. 85 %), suggesting that the prognosis for this variant may be compromised [37]. In contrast, in a SEER study of 172 patients with the Hürthle cell variant and 783 patients with non-Hürthle cell FTC, the histological distinction was less important than patient’s age and gender and tumor stage with regard to association with the 10-year survival rate [38].
Treatment
The appropriate management of MIFC has not reached universal consensus. Based on the low rate of metastases and disease-specific mortality, there are proponents of thyroid lobectomy (with or without isthmusectomy) as an adequate treatment for MIFC [6, 10, 13, 15, 29, 39–41]. In one recent study, 124 patients with FTC were divided into three groups: MIFC, MIFC with vascular invasion, and WIFC. Overall, the disease-free survival rate was 85 % at a median follow-up of 40 months, but disease-free survival differed significantly among the groups, with rates ranging from 97 % for the MIFC group to 81 % for the MIFC withvascular invasion group to 46 % for the WIFC group. Only patients <45 years with MIFC (no vascular invasion) had 100 % disease-free survival, suggesting that in these patients, thyroid lobectomy alone may be sufficient, and that total thyroidectomy [with or without radioactive iodine ablation (RAI)] should be performed in all other patients with FTC [39].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree