Fig. 3.1
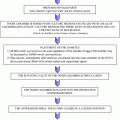
The regulation of oocyte meiotic prophase arrest and resumption in mammals. NPPC produced by mural granulosa cells stimulates the generation of cGMP by NPR2 of cumulus cells. The cGMP then diffuses into oocytes and maintains meiotic prophase arrest by inhibiting oocyte-specific PDE3A activity and cAMP hydrolysis. Intraoocyte cAMP is produced by GPR3/12 activation of ADCY endogenous to the oocyte. Oocyte itself also promotes cumulus cell expression of NPR2 to elevate cGMP levels for meiotic arrest. FSH, through estrogen, enhances NPPC/NPR2 expression to ensure meiotic arrest during antral follicular development. LH-induced EGF-like growth factors decrease NPPC content and NPR2 activity, resulting in cGMP decrease and meiotic resumption. NPPC natriuretic peptide type C; NPR2 natriuretic peptide receptor 2; cGMP cyclic guanosine 3′,5′-monophosphate; cAMP cyclic adenosine 3′,5′-monophosphate; GPR3/12 G-protein-coupled receptor 3 and 12; ADCY adenylyl cyclase; ODPF oocyte-derived paracrine factors; PDE3A phosphodiesterase 3A; FSH follicle-stimulating hormone; LH luteinizing hormone; EGF-like factors, epidermal growth factor-like factors
Oocyte Maturation Inhibitor
In 1935, it was discovered that oocytes or cumulus–oocyte complexes (COCs) could resume meiosis spontaneously without hormonal stimulation when they were liberated from rabbit antral follicles and cultured under simple nutritionally supportive conditions [10]. This original observation is confirmed by numerous studies with most mammalian, including human [11]. Interestingly, the time course of spontaneous maturation is similar to that of LH stimulation in vivo [11]. These observations lead to general acceptance of the hypothesis that the follicular granulosa cells prevent precocious resumption of meiosis until LH initiates oocyte maturation before ovulation. This hypothesis is further corroborated by the studies that co-culture of oocytes with follicular granulosa cells, granulosa cell extract and follicular fluid inhibits oocyte spontaneous maturation [12]. For a long time, many studies focus on identifying the factors participating in the maintenance of meiotic arrest and lead to partial characterization and purification of a factor, oocyte maturation inhibitor (OMI), from follicular fluids [13]. This OMI is a peptide of low molecular weight (~2000 Da), action on cumulus cells without species specificity [12]. The inhibitory effect of OMI can be overcome by the addition of LH, supporting that OMI has a physiological role in the regulation of meiosis [12].
Cyclic Nucleotides
Meiotic arrest depends on a high level of cyclic adenosine 3′,5′-monophosphate (cAMP). Cyclic AMP is produced within oocytes by a constitutive activation of heterotrimeric G-protein (Gs)-coupled receptor GPR3 and GPR12 to stimulate adenylate cyclase (ADCY) [14–18] and is sustained by cyclic guanosine 3′,5′-monophosphate (cGMP) inhibiting cAMP-specific phosphodiesterase 3A (PDE3A) activity in oocytes [19, 20]. Inability to sustain oocyte cAMP concentrations leads to precocious gonadotropin-independent resumption of meiosis, which interrupts the synchrony between oocyte maturation and ovulation and compromises female fertility [15, 16, 21].
Cyclic AMP
Intraoocyte cAMP controls meiosis
Intraoocyte cAMP plays a central role in the regulation of meiosis [22]. The sustained high levels of cAMP are essential for the maintenance of meiotic arrest of fully grown oocytes [1, 23, 24], and a drop in intraoocyte levels of this nucleotide is required for resumption of meiosis [1, 25, 26]. When the oocytes are released from the antral follicle, they resume meiosis spontaneously in parallel with decreases in cAMP levels [27–29]. The pharmacological increase of intraoocyte cAMP levels prevents LH-induced meiotic resumption in vivo [30] and spontaneous maturation in vitro [24, 31, 32].
High levels of cAMP within the oocyte activate protein kinase A (PKA), which in turn phosphorylates (and activates) the kinase WEE1B/myelin transcription factor 1 (MYT1). In addition, PKA-mediated phosphorylation of the phosphatase cell division cycle 25 (CDC25) results in its cytoplasmic retention. The combined action of these two PKA substrates, inhibiting CDC25B and activating WEE1B/MYT1, insures low levels of cyclin-dependent kinase 1 (CDK1) activity, rendering maturation-promoting factor (MPF, a complex of CDK1 and cyclin B) inactive such that the oocyte maintains meiotic arrest [2, 22, 32, 33]. The decrease in oocyte cAMP triggers maturation by alleviating the aforementioned phosphorylations of WEE1B/MYT1 and CDC25B [2]. Meiosis arrest female 1 (MARF1) expressed in oocytes is critical for the activation of MPF, possibly by downregulating the expression of protein phosphatase 2, catalytic subunit, betaisozyme (Ppp2cb) [34].
Cyclic AMP synthesis
A long-standing hypothesis is that cAMP is generated by somatic cells and diffuses into oocytes through heterologous gap junctions between cumulus cells and oocytes [9, 35–37]. Recent studies using knockout mice and microinjection of inhibitory factors confirm that oocyte itself produces sufficient cAMP for meiotic arrest [14–18]. In mouse oocytes, cAMP is produced mainly by GPR3-Gs-ADCY3 pathway. Depletion of GPR3 and ADCY3, or microinjection of inhibitory Gs antibody into follicle-enclosed oocytes, results in precocious resumption of meiosis [14, 15, 38]. However, GPR3 knockout mice are fertile in young animals [21], indicating additional pathway(s) for generation and maintenance of a sufficient cAMP level. In human, GPR3 expressed in oocytes is the predominant receptor signaling for meiotic arrest [39], but in rat, GPR12 is the predominant receptor signaling for meiotic arrest for that downregulation of GPR12 causes meiotic resumption [16].
Taken together, oocyte cAMP is essential for maintaining meiotic arrest and is generated by oocyte ADCY, which is produced by the constitutive action of GPR3 and GPR12 via Gs protein [16, 32]. Although the transfer of cAMP from surrounding cumulus cells to oocytes is possible, it is not sufficient by itself to maintain meiotic arrest [32, 40]. Currently, the natural ligand(s) for GPR3 and GPR12 remain unknown. It has been reported that the GPR3 is most closely related to lipid and peptide receptors [41]. Furthermore, incubation of mouse oocytes with sphingosine 1-phosphate (S1P) and sphingosylphosphorylcholine (SPC) has been shown to delay spontaneous oocyte maturation [16], indicating that a lipid may stimulate GPR3 and GPR12.
Cyclic AMP degradation
Intraoocyte cAMP levels depend on the synthesis by GPR3/12 and the degradation by an oocyte-specific phosphodiesterase (PDE) 3A [15, 30, 42–45]. Maintenance of meiotic arrest is associated with undetectable cAMP-PDE activity [31, 46, 47], and inhibition of PDE3 activity elevates intraoocyte cAMP and prevents the resumption of meiosis in many species including human [2, 48]. Moreover, genetic ablation of PDE3A causes complete meiotic arrest either after an LH surge or COCs culture in vitro and female sterility [47, 49]. Depleting both of GPR3 and PDE3A genes allows spontaneous meiosis resumption in vivo as depletion of GPR3 alone, and the increases in cAMP levels have not been detected in oocytes isolated from those double-knockout mice [40], suggesting that PDE3A is downstream of GPR3 in regulating intraoocyte cAMP levels. These studies demonstrate that intraoocyte cAMP levels are regulated via control of PDE3A-mediated degradation, rather than endogenous synthesis. Inhibition of PDE3A activity is essential for sustaining elevated cAMP levels that maintain meiotic arrest, and activation of PDE3A is required to promote the cAMP degradation that initiates meiotic resumption.
Cyclic GMP
As early as 1980, it is found that cGMP levels are highest at diestrus but lowest during estrus or LH stimulation in hamster ovary [50], suggesting a functional relationship between cGMP levels and meiosis. Many studies show that cGMP is involved in the regulation of oocyte maturation. The blockade of inosine monophosphate dehydrogenase (needed for cGMP production) causes meiotic resumption in follicle-enclosed oocytes [51]. Intraoocyte cGMP levels decrease during oocyte spontaneous maturation [27], but increase of cGMP levels by 8-Br-cGMP or cGMP-specific PDE5 inhibitor suppresses meiotic resumption [28, 52, 53].
The microinjection of a cGMP-specific PDE5 into oocytes causes meiotic maturation of wild-type oocytes, but this effect is absent in PDE3A-deficient oocytes [20], suggesting the inhibitory effect of cGMP on oocyte maturation through the regulation of PDE3A activity. It is reported that cGMP has an inhibitory effect on PDE3A activity. Cyclic GMP and cAMP-binding regions in PDE3A are overlapping but not identical [54], and GMP inhibits PDE3A activity via completion with cAMP in the hydrolysis process [55]. The concentration of cGMP in GV-stage oocytes isolated from equine chorionic gonadotropin (eCG)-primed immature mice is sufficient to inhibit PDE3A activity [20, 27, 54]. Guanylyl cyclase agonists have inhibitory effects on spontaneous meiotic resumption in COCs, but not in isolated oocytes [28, 56], suggesting that the oocyte depends on the somatic cells for its supply of cGMP. Further studies showed that cGMP, produced by somatic cells, diffuses through the gap junction network to the oocyte and inhibits PDE3A activity [19, 20]. This inhibition sustains a high level of cAMP in the oocyte for meiotic arrest. Thus, the production of cGMP in the somatic cells is a critical component required to maintain prophase I arrest.
NPPC
Cyclic GMP can be produced by two distinct classes of guanylyl cyclases, soluble and particulate, activated by nitric oxide (NO) and natriuretic peptides, respectively [57–59]. NO is a chemical messenger enzymatically produced by three isoforms of nitric oxide synthases (NOS): endothelial (eNOS), neuronal NOS (nNOS) and inducible isoform (iNOS) [59]. Although these three isoforms of NOS have been detected in mammalian ovaries [60], a high concentration of NO donor sodium nitroprusside (1 mM) has an inhibitory effect on mouse oocyte spontaneous maturation [61]. However, knockouts of Nos1 and Nos2 affect ovulation [62, 63], and knockout of Nos3 appears to impair oocyte development [64]. The role of NO appears to be mainly in the control of ovulation and not in the regulation of meiotic arrest.
The natriuretic peptide system forms a family of three structurally homologous but genetically distinct endogenous ligands: type A (NPPA, also known as ANP), type B (NPPB, also known as BNP) and type C (NPPC, also known as CNP) [57]. In general, NPPA and NPPB activate particulate guanylate cyclase natriuretic peptide receptor 1 (NPR1, also known as GC-A, NPRA), and NPPC activates receptor NPR2 (also known as GC-B, NPRB) [65]. NPPA and NPPB are cardiac hormones that are predominantly synthesized in atrial and ventricular cardiomyocytes, respectively, and play important roles in the regulation of cardiovascular homeostasis [65]. NPPA is reported to slightly inhibit spontaneous meiotic resumption of rat oocytes [28]. However, Nppa and Nppb mRNA could not be detected by in situ hybridization [66] and by specific riboprobes in mouse ovary [67]. Also, the expression of Npr1 transcription in cumulus cells is very low by real-time PCR analysis [66]. All these results indicate that NPPA, NPPB and their receptor NPR1 do not seem to be the crucial mechanism of meiotic arrest.
NPPC, on the contrary, is expressed in a wide variety of central and peripheral tissues and acts locally as autocrine and paracrine regulator but little natriuretic activity [68]. NPPC and its guanylyl cyclase receptor NPR2 are present in rat granulosa cells and show coordinate estrous cycle-dependent variation with maximal expression at proestrus [69]. Further studies show that Nppc mRNA is expressed predominantly by mouse MGCs, which line the inside of the follicular wall, and, in contrast, Npr2 mRNA is expressed predominantly by cumulus cells surrounding the oocyte [70]. Application of NPPC to the culture medium prevents spontaneous meiotic resumption of oocytes that are surrounded by cumulus cells, and increases intraoocyte cGMP and cAMP levels [70]. NPPC has no inhibitory effect on denuded oocytes for the lack of NPR2 receptors. Importantly, meiosis resumes precociously in oocytes within antral follicles of Nppc and Npr2 mutant mice [70, 71]. Disruption of gap junctions by isoform-specific connexin mimetic peptides indicates that both connexin-43 (GJA1) and connexin-37 (GJA4) gap junctions are required for NPPC-mediated meiotic arrest [72]. Thus, NPPC produced by follicular MGCs stimulates the generation of cGMP by cumulus cells NPR2, which diffuses into oocyte via gap junctions and maintains meiotic arrest by inhibiting PDE3A activity and cAMP hydrolysis [20, 70, 73, 74]. In mammals, meiotic maturation of oocytes must be coordinated precisely with ovulation to produce a developmentally competent egg at the right time for fertilization. Therefore, NPPC/NPR2 stimulates production of cGMP for preventing premature meiosis in oocytes, which is critical for maturation and ovulation synchrony and for normal female fertility. Inappropriate decrease of NPPC and NPR2 in the growing follicles reduces oocyte developmental capacity and so fertility [21, 75, 76].
For a long time, it has been suggested that MGCs original OMI, acting on cumulus, maintains meiotic arrest. It is interesting that MGC-derived NPPC has the character similar to the OMI: a 22 amino acid residues peptide with molecular weight of 2197.6, action via NPR2 by cumulus cells, and identical sequences among mouse, rat, pig and human [70, 77]. NPPC from human shows the inhibitory effect on the maturation of oocytes from mouse [70], rat (unpublished data), pig [78, 79] and cattle [76]. Furthermore, LH can overcome the inhibitory effect of NPPC [80], supporting the concept that NPPC may be the ‘OMI’ responsible for meiotic arrest in mammal oocytes. It is reported that porcine NPPB (pNPPB) shows a high affinity for NPR2 in the cells from pig, human, rat and mouse to produce the functional effect [65, 81]. pNPPB also shows a inhibitory effect on spontaneous oocyte maturation of pig and mouse [66, 78, 79]. Clearly, this relationship between pNPPB and native or non-native NPR2 activation needs further study. A better understanding of the factors that maintain an oocyte in meiotic arrest may help in the development of strategies to improve culture conditions with particular regard to the quality of cytoplasmic maturation [76, 82, 83].
FSH
As noted above, NPPC/NPR2-produced cGMP is essential for maintaining meiotic arrest of oocytes within antral follicles. Thus, stimulating the expression of NPPC/NPR2 during follicular development is required to maintain oocyte meiotic arrest. During each estrous cycle, some early antral follicles are ‘recruited’ by follicle-stimulating hormone (FSH) stimulation from the pituitary to continue growing and develop into preovulatory antral follicles (Graafian follicles) [84, 85]. Interestingly, NPPC/NPR2 levels in rat ovary vary during the estrous cycle and are maximal at proestrus [69]. The treatment of equine chorionic gonadotropin (eCG), a glycoprotein hormone that possesses primarily FSH activity, stimulates Nppc mRNA expression in mouse MGCs and increases NPPC content in the ovaries [80, 86–88]. The expression of Npr2 mRNA in both MGCs and cumulus cells is also increased after eCG treatment [86, 87]. However, FSH cannot stimulate the expression of Nppc and Npr2 mRNA in MGCs and cumulus cells cultured in vitro [87, 88], suggesting that FSH activity of eCG induces the expression of NPPC/NPR2 indirectly. The distinct physiological action of FSH is to stimulate follicular growth [89] although FSH can stimulate oocyte maturation of COCs cultured in vitro [53, 90]. Thus, the increasing expression of NPPC/NPR2 insures their ability to prevent oocyte precocious maturation during FSH-stimulated antral follicular development. Inappropriate expression of NPPC/NPR2 in the growing follicles may disrupt meiotic arrest and normal follicular development [91, 92].
Estrogen
Mammalian follicular development is associated with increased production of estrogen by MGCs through FSH or eCG-stimulated aromatization of testosterone [89], which partially mediates FSH action [93–95]. The synthetic estrogen diethylstilbestrol (DES) stimulates Nppc and Npr2 mRNA expression in rat ovary in vivo [96]. In vitro, 17β-estradiol (E2) raises the levels of Nppc and Npr2 mRNA in cultured mouse MGCs [88]. E2 also promotes expression of Npr2 mRNA by cumulus cells, thereby augmenting NPPC ability to produce cGMP and maintain meiotic arrest of COCs cultured in vitro [87]. E2-promoted Nppc mRNA expression can be enhanced by interaction with FSH [88]. Testosterone promotes Npr2 mRNA expression by cumulus cells of cultured COCs possibly due to aromatization of testosterone to estrogens [87]. All these results implicate the physiological role of estrogens is involved in maintaining oocyte meiotic arrest through inducing the expression of NPC/NPR2. However, there is no indication from published reports that the oocytes within antral follicles show precocious resumption of meiosis after estrogen receptors or Cyp19α1 (aromatase) deletions [97–101]. Thus, other pathways could participate in compensation for the absence of estrogens, in the mechanisms maintaining meiotic arrest in vivo. Optimal fertility requires synchrony in the regulation of oocyte meiotic events and ovulation [15, 16, 21]. It would not be surprising, therefore, if compensatory or redundant mechanisms evolved into interacting pathways that maintain meiotic arrest and assure this essential synchrony.
Oocyte-Derived Paracrine Factors
An increasing body of evidence indicates that MGCs have important endocrine functions, and oocyte-derived paracrine factors (ODPFs) profoundly affect the differentiation of cumulus cells [102]. Higher expression of Npr2 mRNA in cumulus cells than in MGCs implies that ODPFs promote its expression [102]. Indeed, removing oocytes from follicles reduces Npr2 mRNA expression in cumulus cells, but culturing these cumulus cells with denuded oocytes restores its expression [70]. Many studies have focused on oocyte-secreted transforming growth factor-β (TGF-β) superfamily members, in particular growth differentiation factor 9 (GDF9), bone morphogenetic protein 15 (BMP15; also called GDF9B) and fibroblast growth factor 8B (FGF8B) [103]. Each of these three ODPFs slightly promotes expression of Npr2 mRNA by cumulus cells in vitro, and combinations of three proteins restore Npr2 mRNA expression in isolated cumulus cells [18, 70]. It is surprised that ODPFs also stimulate Nppc mRNA expression in cumulus cells [88] although the levels of Nppc mRNA in cumulus cells are very lower compared with that in MGCs [70]. Although ODPFs can promote the production of estradiol by cumulus cells [104, 105], it is unlikely that ODPFs promote NPPC/NPR2 expression in cumulus cells by estradiol [87]. ODPFs are suggested to act on cumulus cells by the activation of Smad (Sma and Mad-related protein) signaling pathway [102, 106]. Knockout of Smad4, the central component of the canonical TGF-β signaling pathway, reduces Nppc and Npr2 expression in both MGCs and cumulus cells, and maintaining oocyte meiotic arrest is weakened [92]. All these results suggest that ODPFs-induced NPPC/NPR2 expression plays an important role in maintaining meiotic arrest of oocytes within antral follicles, especially when the estradiol signal is absent or reduced.
NPPC/NPR2 system requires the activity of inosine monophosphate dehydrogenase (IMPDH), the rate-limiting enzyme required for the production of guanylyl metabolites and cGMP. ODPFs, particularly the GDF9-BMP15 heterodimer, also promote expression of IMPDH and elevate cGMP levels in cumulus cells that required for meiotic arrest [107]. Thus, oocytes themselves contribute to meiotic arrest by generating cAMP via GPR3/12 and maintaining cAMP levels via promoting expression of NPPC/NPR2 and IMPDH to elevating cGMP levels in cumulus cells. These results support the view that the signals originating from the oocytes play an essential role in orchestrating follicular growth and development [103].
Meiotic Resumption
LH
Fully grown mammalian oocytes within Graafian follicles are held in meiotic prophase arrest by NPPC/NPR2-produced cGMP. The preovulatory surge of LH from the pituitary gland triggers meiotic resumption during the estrous or menstrual cycle. The LH signal is amplified by promoting the production of epidermal growth factor (EGF)-like growth factors in MGCs and then the transactivation of the EGF receptor (EGFR) in cumulus cells, by which LH reduces NPPC content in the follicle and NPR2 activity in cumulus cells, and the resulting cGMP decrease and meiotic resumption.
Reduction of Intraoocyte cAMP Levels
In a normal reproductive cycle, the preovulatory surge of LH from the pituitary acts on the granulosa cells of Graafian follicles to cause oocyte maturation and ovulation [2]. However, the precise mechanisms underlying the LH-induced oocyte maturation are not completely understood [45, 108]. LH receptor activation stimulates Gs and activates adenylyl cyclase [109] and, as a consequence, elevates cAMP levels in the MGCs [110, 111]. Through a series of incompletely understood steps, LH ultimately causes a decrease in cAMP in the oocyte that is required for meiotic resumption [110, 112, 113]. LH induced the decrease of intraoocyte cAMP levels either by the reduction of cAMP synthesis or an increase in cAMP hydrolysis. LH-induced signaling does not terminate GPR3/12-Gs-ADCY signaling [73] or stimulate a Gi-mediated pathway in the oocyte [114]. On the contrary, the increase in oocyte-specific PDE3A activity after LH surge is likely sufficient to decrease cAMP levels in oocytes and thereby initiates pathways governing meiotic resumption [89].
Reduction of Intraoocyte cGMP Levels
PDE3A activity is regulated by intraoocyte cGMP levels. LH surge results in the decrease of intraoocyte cGMP levels [89]. The reduction of cGMP levels in oocytes relieves the inhibition of PDE3A, cAMP hydrolysis and meiotic resumption [19]. LH stimulation could not induce oocyte maturation under the conditions of elevated intraoocyte cGMP levels [20]. The reduction of intraoocyte cGMP levels is caused by lowering cGMP levels in the somatic cells and/or by closing the heterologous gap junctions coupling the somatic cells and oocytes [19, 20]. Gap junctions play an important role in signaling between somatic cells and oocyte. Connexin-43 is the predominant connexin present in granulosa cells, whereas connexin-37 is the major connexin present in junctions between the cumulus cells and oocyte [115, 116]. LH induces the phosphorylation of connexin proteins via the activation of extracellular signal-regulated kinases 1 and 2 [ERK1/2, also known as mitogen-activated protein kinases 3 and 1 (MAPK3/1)] [74, 111, 117, 118], by which LH decreases the permeability of gap junctions to reduce cGMP flux from somatic cells into oocytes [45]. Although the pharmacological closure of gap junctions is sufficient to initiate meiotic resumption of follicle-enclosed oocytes [113], studies in human, porcine, ovine and murine oocytes suggest meiotic resumption precedes the closure of gap junctions [18, 45, 119]. Furthermore, blocking the closure of gap junctions using the REK1/2 inhibitor U0126 (10 μM) does not prevent LH-mediated meiotic resumption [74]. These results indicate that the decrease of intraoocyte cGMP levels primarily contributes to LH-induced cGMP reduction in the somatic cells [80].
The decrease of cGMP levels in the somatic cells could result from the reduction in cGMP synthesis and an increase in cGMP degradation. There is some evidence for regulation of the guanylyl cyclase rather than the cGMP-PDEs [120]. The increased cGMP-PDEs activity has not been detected in mouse and rabbit ovaries during LH stimulation [20, 120], and human chorionic gonadotropin (hCG) has no effect on cGMP hydrolysis in rat granulosa cells in vitro [121]. In the presence of cGMP special PDE5 inhibitor, LH still decreases cGMP levels [20], suggesting that LH acts by decreasing cGMP synthesis in somatic cells rather than increasing cGMP-PDEs activity [120]. Recent study shows that EGF receptor-dependent events are involved in the short-term regulation of cGMP, whereas the long-term effects may involve regulation of the NPPC [122]. It is reported that LH-induced ERK1/2 activation could rapidly inhibit the expression of Cyp19α1 mRNA, which encodes aromatase, and so decrease estradiol levels [123–125], by which LH may negatively regulate the expression of NPPC/NPR2 in somatic cells to decrease cGMP levels.
Reduction of NPPC/NPR2 Function
LH decreases cGMP levels in the somatic cells by decreasing the function of NPPC/NPR2 [80, 120]. The activation of LH receptors by hCG, a pregnancy hormone that exhibits LH activity with a long serum half-life, decreases Nppc mRNA levels in MGCs by approximately half of basal levels within 2 h before GVB occurs [80, 86, 88, 126]. This could result in a rapid decrease in the amount of NPPC, since NPPC has a half-life of approximately 3 min in plasma [127]. It is also possible that increased protease activity results in the degradation of NPPC [128]. Although LH receptor activation increases the expression of Npr3 mRNA in MGCs [88] and NPR3 agonist enhances NPPC-mediated meiotic arrest of porcine oocyte cultured in vitro [129], an NPPC clearance receptor, NPR3, probably does not participate in regulation of ovarian NPPC levels for that there is no effect on hCG-induced NPPC decrease in Npr3 mutant mice [88]. Nevertheless, NPPC peptide levels are completely decreased in mouse and human ovaries after the activation of LH receptors [80, 86], which occurs early enough to potentially contribute stimulation of nuclear envelope breakdown [80].
LH receptor signaling also decreases Npr2 mRNA levels in cumulus cells [86]. However, this decrease occurs approximately 3 h after hCG stimulation when most oocytes have resumed meiosis [87]. On the other hand, LH could induce meiotic resumption of oocytes within cultured follicles even in the presence of 100 nM NPPC (unpublished data). It is suggested that NPR2 guanylyl cyclase activity can be decreased in a manner that is independent of its protein levels [130, 131]. Consistent with this, LH treatment for 20 min decreases NPR2 activity of approximately 50% in MGCs without the change of NPR2 protein levels, resulting in the rapid reduction in follicle cGMP levels [80]. The rapid decrease in NPR2 activity can be caused by dephosphorylation [128, 132].
Thus, LH decreases NPPC content and NPR2 activity, each of which is enough to induce oocyte maturation by decreasing cGMP levels in the somatic cells and then in oocytes [80, 133]. A decline in oocyte cGMP results in increased PDE3A activity, cAMP hydrolysis and meiotic resumption [49]. It has been long hypothesized that the action of LH could either relieve a maturation-arresting substance from the somatic cells or alternatively provide a positive maturation-promoting substance to override the follicular inhibition [1, 23]. Above data are consistent with a model in which LH removes the inhibitory function of NPPC/NPR2 to subsequently trigger oocyte maturation. It will be of interest to examine the exact mechanisms by which LH decreases NPPC content and NPR2 activity.
EGF-Like Growth Factors
LH receptor exists in MGCs, but not in cumulus cells [134]. LH stimulation triggers synthesis of EGF-like growth factors amphiregulin (AREG), epiregulin (EREG) and betacellulin (BTC) in MGCs [135], which is essential to transmit the LH signal from the MGCs to the cumulus cells to induce oocyte maturation and ovulation [111, 135–137]. AREG and EREG are the primary signaling molecules synthesized in response to LH to induce oocyte maturation [135]. Oocytes from both AREG and EREG knockout mice display a significant delay in the onset of meiotic maturation after the LH surge in vivo [111]. The disruption of ERK1/2 in mouse granulosa cells impairs hCG-induced generation of EGF-like growth factors [138], suggesting that the activation of ERK1/2 is involved in the production of these growth factors. EGF-like growth factors are produced as transmembrane precursors and release soluble growth factors after cleaved at cell surface by extracellular proteases. These factors trigger tyrosine-kinase EGF receptor (EGFR) signaling on the target cells leading resumption of meiosis [18, 45, 109, 135]. Activation of EGFR, as indicated by increased phosphorylation of the receptor protein, occurs as early as 30 min after LH treatment [137, 139], and LH-induced resumption of meiosis is strongly inhibited in AREG −/− EGFR wa2/wa2 mice [111].
The activation of EGFR by amphiregulin and EGF rapidly suppresses Nppc mRNA levels within 2 h in cultured granulosa cells [71, 88]. However, this EGFR-mediated decrease of Nppc mRNA is about half of control. On the other hand, the EGFR inhibitor AG1478 incompletely inhibits LH-induced decrease of Nppc mRNA and cGMP levels [20, 117, 140]. These findings suggest that EGFR activity is not required for all of the LH-induced NPPC decrease, and two separate and partially redundant mechanisms contribute to the decrease of NPPC content in response to LH. The activation of the EGFR also decreases the levels of Npr2 mRNA in cumulus cells cultured in vitro [133]. However, this decrease may not be involved in EGF-induced meiotic resumption, since the blockade of Npr2 mRNA decrease by 10 μM U0126 could not reverse EGF-induced meiotic resumption [133]. The inhibition of NPR2 protein de novo synthesis by cycloheximide has also no effect on EGF-mediated oocyte maturation [133], suggesting that nascent gene transcription is not required in this process [141, 142]. EGF overcomes NPPC-mediated inhibition of maturation of oocytes in cultured COCs by decreasing cGMP levels, but has no effect on oocyte maturation when meiotic arrest is maintained in the presence of cGMP analog 8-bromoadenosine-cGMP [133]. Thus, LH stimulation of EGF-like growth factors in MGCs activates EGFR transactivation in cumulus cells, which is essential for meiotic resumption by the reduction of NPR2 activity of cumulus cells [22, 109, 135, 139]. The activation of EGFR in cumulus cells also causes phosphorylation of AKT and mTOR activation in oocytes, resulting in an increase in translation of a subset of maternal mRNAs. These mRNA translations are essential to reprogram the oocyte for embryo development [143].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree