Fig. 31.1
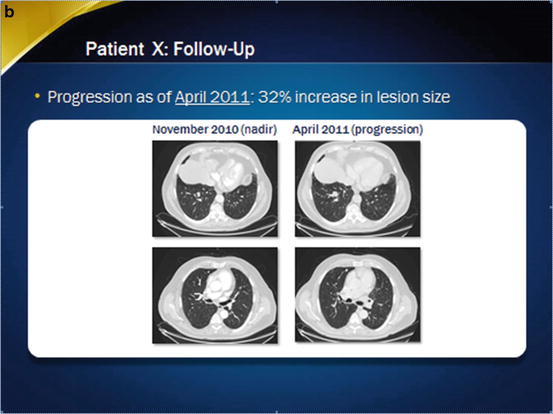
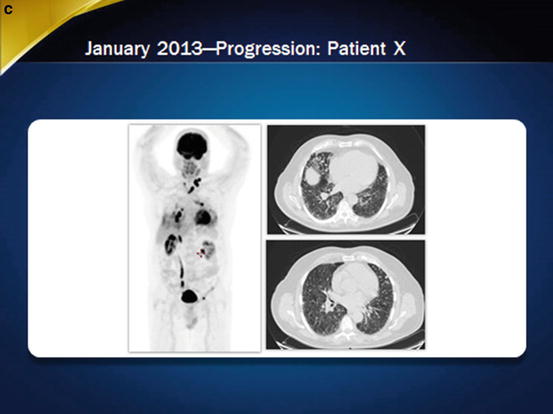
Imaging of the patient at baseline and during sorafenib treatment. In November 2010, a partial response was observed (panel a), but thereafter, in April 2011, the patient progressed despite treatment (panel b). Less than 2 years after treatment withdrawal, in January 2013, progression was obvious (panel c), and the patient initiated a second-line treatment
In February 2011, the daily dose of sorafenib was decreased to 600 mg for painful hand-foot skin reaction (grade 3) that was no longer controlled by local treatment modalities, diarrhea (grade 2), and fatigue (grade 2).
Symptoms improved and the patient continued to receive sorafenib 600 mg a day. In April 2011, progression was observed with a 32 % increase in lesion size from the nadir (Fig. 31.1b). Treatment with sorafenib was discontinued, and the patient refused a different second-line systemic therapy.
After withdrawal of sorafenib, disease progressed at a pace similar to that observed before treatment (Fig. 31.1c). In May 2012, 12 months after discontinuation of sorafenib treatment, dyspnea occurred, indicating the need for additional treatment, but the patient refused again for personal reasons. Dyspnea progressively worsened and was accompanied by hemoptysis. In March 2013, a bronchoscopy showed a lesion in the left mainstem bronchus. Since bronchial invasion increases the risk of bleeding, enrollment in another clinical trial was not possible. Testing for BRAF and ALK mutations was performed because the presence of a driver mutation may lead to a specific inhibitor, but this was negative.
The tumor board decision was that second-line treatment should not be delayed, and the patient agreed to additional therapy. In May 2013, treatment with off-label sunitinib 37.5 mg/day for 3 weeks followed by 1 week off therapy was initiated. After two cycles, dyspnea improved and he had no hemoptysis. In August 2013, a CT scan demonstrated a partial tumor response (−35 %) using Response Evaluation Criteria in Solid Tumors (RECIST). He had minor adverse events, consisting in fatigue (grade 1) and intermittent diarrhea (grade 1) and enjoyed an almost normal quality of life (ECOG 0). Sunitinib treatment was maintained, and chest CT scans performed every 3 months did not show tumor progression. In December 2014, the patient was still receiving sunitinib treatment at the same dosage with no significant adverse events and a good quality of life.
Discussion
Radioiodine-refractory thyroid cancer is uncommon, with an estimated incidence of 4–5 cases per million population (5 % of patients with clinical thyroid cancer, 250 patients per year in France) [1, 2]. It occurs more frequently in older patients, in those with large metastases or with poorly differentiated thyroid cancer, and in those with high FDG uptake on PET scan [3–5].
Definition of 131I-Refractory Thyroid Cancer
Most patients with 131-I-refractory DTC fall into four categories [6]: (a) Patients with metastatic disease that does not take up 131-I at the time of the first 131-I treatment. (b) Patients whose tumors lose the ability to take up 131-I after previous evidence of uptake. This is due to the eradication by 131-I treatment of differentiated cells able to take up 131-I, but not of less differentiated cells that do not take up 131-I and that are likely to progress. (c) Patients with 131-I uptake retained in some lesions but not in others. This is frequently observed in patients with multiple large metastases. In such patients, progression is likely to occur in metastases without 131-I uptake (in particular when FDG uptake is present) [3, 5, 7]. (d) Patients with metastatic disease that progresses despite significant uptake of 131-I in all the metastases and adequate 131-I treatment. It has been shown that if progression occurs following a course of adequate radioiodine treatment, subsequent 131-I treatment will be ineffective [8].
Our patient had lung metastases with no uptake on posttherapy WBS. There was an increase in serum TSH level following thyroid hormone withdrawal, and there was no iodine contamination; therefore, technical conditions did not prevent visualization of potential uptake at distant sites. Thus, the patient was considered 131-I refractory and no further RAI was administered. This is consistent with the age of the patient (70 years) and with the presence of FDG uptake in some metastases on PET/CT.
Treatment Initiation
Patients with advanced disease who are refractory to 131-I treatment have a median survival rate of 3–6 years in the absence of effective treatment modalities and a 10-year survival rate of only 10 % [4]. However, patients with refractory thyroid cancers encompass a heterogeneous group with regard to progression rate and life expectancy. For example, more aggressive tumors occur in older patients, are poorly differentiated, and have no initial RAI uptake but a high FDG uptake, and usually large metastases are present. In contrast, young patients with non-radioiodine-avid small lung metastases from a well-differentiated thyroid carcinoma can be asymptomatically stable for long periods of time. However, there are exceptions, and the rate of progression should be documented by imaging in each patient.
Once 131-I treatment is abandoned, levothyroxine treatment is used to maintain serum TSH at a low or undetectable level, depending on other patient comorbidities, and local treatment of metastases is performed whenever needed. Surveillance includes a FDG-PET/CT scan or a CT scan of the neck, chest, abdomen, and pelvis with contrast medium, at an interval of 3–6 months. In the absence of documented progression, follow-up with anatomical imaging every 6–12 months is continued.
The decision to initiate systemic therapy in patients with 131-I-refractory thyroid cancer is based on the presence of a large tumor burden, evidence of disease progression, the presence of symptoms, a high risk of local complications, and the general condition of the patient [1]. Treatment should be initiated preferably before the occurrence of invasion of the respiratory-digestive axis or of encasement of large vessels that increases the risk of severe bleeding during treatment. Some patients may require treatment of local tumor extension with surgery or radiotherapy to permit treatment with kinase inhibitors [9].
The progression rate can also be evaluated by the doubling time of serum Tg [10] but should always be confirmed by anatomic imaging using RECIST criteria. Patients with multiple lesions >1–2 cm and with RECIST progression (i.e., at least a 20 % increase in the sum of the diameters of target lesions or the appearance of significant new metastatic lesions) within less than 12 months are considered for systemic treatment. In contrast, patients with few and/or small metastatic lesions <1 cm or those with no evidence of progression over a 12-month period are followed without treatment, and stable disease or slow progression has been observed in some patients over decades [1, 4].
Our patient had stable disease at 5 months postthyroidectomy, but disease progression was documented within 11 months, with an increase in tumor diameters by 44 % and with the appearance of new lesions. At that point, the sum of the diameters of the largest lesion was >2 cm. The thyroid tumor board concluded that he fulfilled all criteria for treatment initiation. Because of the poor efficacy of cytotoxic chemotherapy [11], the use of tyrosine kinase inhibitor was recommended as first-line treatment.
Molecular-Targeted Therapy
Gene rearrangements (RET/PTC and NTRK) or point mutations of the RAS and BRAF genes are found in two-thirds of papillary thyroid cancers, resulting in the activation of the MAP kinase pathway [2, 12]. An even greater frequency of mutations is observed using next-generation sequencing [13]. Angiogenesis is activated in thyroid cancers, with an overproduction of VEGF by cancer cells and an overexpression of VEGF receptors by cancer and endothelial cells, and is associated with a poor outcome. Furthermore, other pathways such as the fibroblast growth factor receptor (FGFR) and platelet-derived growth factor receptor (PDGFR) pathways may also be activated. Up to now, antiangiogenic drugs, some of which also target kinases in the MAP kinase pathway, have been used in refractory thyroid cancers. The relative role of the inhibition of each target or of their combined inhibition on tumor response is currently unknown.
Results of Clinical Trials with Antiangiogenic Drugs
In phase 2 trials with these agents, partial responses were observed in 0–59 % of patients and long-term stable disease in at least another third. The comparison of the outcomes among these compounds is at the present time not possible, but 50 % or even higher response rates that have been recently reported with pazopanib [14], lenvatinib [15], and cabozantinib [16] seem higher than previously reported with axitinib [9, 17], motesanib [18], sorafenib [19], sunitinib [20], and vandetanib [21]. It also appears that most drugs are more effective in treating metastases located in the lymph nodes, liver, and lungs rather than in the skeleton.
The improvement in progression-free survival (PFS) with these agents is also significant when compared with placebo. This was reported in a randomized phase 2 trial (vandetanib vs. placebo) and in two phase 3 trials (sorafenib vs. placebo and lenvatinib vs. placebo). The lack of demonstrated improvement in overall survival versus placebo in all of these trials might have been the result of the crossover design of these randomized studies.
The ZACTHYF study was a multicenter randomized phase 2 trial with vandetanib (300 mg/day) versus placebo on 145 patients with RAI-refractory, locally advanced, or metastatic DTC that had progressed within the past 14 months. Vandetanib significantly prolonged PFS compared with placebo (hazard ratio, 0.63, p = 0.008; median, 11.1 vs. 5.9 months), but no objective response in lesion size was observed [21].
The DECISION trial was a multicenter, randomized (1:1) phase 3 trial of sorafenib (400 mg twice daily) versus placebo in 417 patients with RAI-refractory, locally advanced, or metastatic DTC that had progressed within the past 14 months [19]. Median treatment duration was 10.6 months (range, 0.07–31.1) with sorafenib and 6.5 months (range, 0.4–30.4) with placebo. The mean daily study sorafenib dose was 651 mg. Sorafenib treatment significantly improved PFS compared with placebo (hazard ratio, 0.587; 95 % CI 0.454–0.758; p < 0.0001; median 10.8 versus 5.8 months, respectively), and the partial response rate was 12 %. The improvement in PFS was seen in all clinical and biomarker subgroups, irrespective of BRAF and RAS mutation status. Median thyroglobulin levels rose in the placebo group and decreased and then paralleled progression in the sorafenib-treated group. The safety profile of sorafenib was as expected, with most adverse events being grades 1 and 2. The most common adverse events in the sorafenib arm were hand-foot skin reaction (76 %), diarrhea (69 %), alopecia (67 %), and rash/desquamation (50 %). Toxicities led to dose reduction in 64 % of patients and to drug withdrawal in 19 %. These results led to the approval of sorafenib by the US FDA for advanced, refractory, and progressive DTC in November 2013 and by the EMA in March 2014.
The SELECT trial was a multicenter, randomized (2:1) phase 3 study of lenvatinib (24 mg/day) versus placebo in 392 patients with RAI-refractory, locally advanced, or metastatic DTC that had progressed within the past 13 months, as confirmed by independent radiological review [1]. Median treatment duration was 13.8 months (range, 0–27) with lenvatinib and 3.9 months (range, 0–24) with placebo. The mean daily study lenvatinib dose was 16.8 mg, with a median time to first dose reduction of 3 months. Lenvatinib treatment significantly improved PFS compared with placebo (hazard ratio, 0.21; 99 % CI, 0.14–0.31, P < 0.001; median PFS , 18.3 vs. 3.6 months, respectively). At year 2, progression events occurred in 86 % of those in the placebo arm and only in 41 % of subjects in the treatment arm. The objective response rate was 65 % with complete responses in 2 %, with a median time to objective response of 2 months. Similar benefits were observed in 20 % of patients who had received prior VEGF-targeted therapy. The improvement in PFS was seen in all clinical and biomarker subgroups, irrespective of BRAF and RAS mutation status. Treatment-related adverse events were reported in all patients in the lenvatinib group. Most often, these were hypertension (68 %), fatigue (64 %), diarrhea (59 %), and decreased appetite (50 %). Proteinuria occurred in 32 % of the patients and thromboembolic events in 11 % of the patients. These adverse events were managed with dose modification and medication. Toxicities led to dose reduction in 68 % of the patients and to drug withdrawal in 14 % of patients. In the active treatment arm, there were 20 fatalities compared with six in the placebo arm. Investigators attributed six fatalities (2 %) directly to the use of lenvatinib. One person died from a pulmonary embolism, one died due to hemorrhagic stroke, and the other four patients died due to general health deterioration. In conclusion, lenvatinib seems more effective than any other kinase inhibitor with an almost 15-month improvement in median PFS compared with placebo and a response rate as high as 65 %, with few complete responses. However, efficacy and toxicity of lenvatinib have still to be evaluated in real life, outside the frame of a controlled trial.
Metabolic consequences of kinase inhibitor treatment are relevant in DTC patients. An increased need of levothyroxine is frequent, and serum TSH should be measured at each visit. An increased need for calcium and vitamin D may occur, particularly in patients with postoperative hypoparathyroidism, and ionized calcium should be measured at each visit during treatment and also when kinase inhibitor treatment is withdrawn to avoid severe hypercalcemia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree