© Springer Science+Business Media New York 2015
Ashraf Khan, Ian O. Ellis, Andrew M. Hanby, Ediz F. Cosar, Emad A. Rakha and Dina Kandil (eds.)Precision Molecular Pathology of Breast CancerMolecular Pathology Library1010.1007/978-1-4939-2886-6_44. Molecular Pathology of Precancerous Lesions of the Breast
(1)
Division of Cancer and Stem Cells, Department of Histopathology, School of Medicine, University of Nottingham and Nottingham University Hospitals NHS Trust, Nottingham City Hospital, Nottingham, UK
Keywords
Molecular pathologyPrecancerous lesionsBreast cancerLow gradeHigh gradeGeneticsIntroduction
Evidence has now emerged that low- and high-grade breast cancers (BCs) evolve through distinct evolutionary pathways and not through various steps of dedifferentiation [1–4] and differences between these tumours are maintained throughout the process of tumour initiation, development and progression. This is reflected in the molecular biology of precancerous lesions of the breast, which includes the low-grade lesions, as part of the ‘low grade neoplasia family’, as well as the high-grade lesions. At the lower end of the spectrum, the coexistence of low-grade precursor lesions with invasive low-grade BCs that defy justification by chance alone [5, 6] as well as their overlapping morphological, and immunohistochemical and genetic features [5–9] provide evidence that these lesions represent a continuum in terms of BC development and progression. Their low proliferative activity and characteristic features are fundamentally different from those observed in precursors of a histological higher grade BC [6]. When high-grade precursors progress to invasive cancer, the latter is usually of high-grade, sharing the same genetic aberrations. This chapter explores the fundamental differences in molecular profiles of the two spectrums of precancerous lesions of the breast, low and high grade.
Low-Grade Precursor Lesions
Salient Histopathological Features
These clonal intraductal lesions originate from the terminal duct-lobular unit (TDLU) and show low-cytonuclear atypia with or without intraluminal proliferation (summarised from [10, 11]). Columnar cell lesion (CCL), flat epithelial atypia (FEA), atypical ductal hyperplasia (ADH), atypical lobular hyperplasia (ALH), lobular carcinoma in situ (LCIS) and low-grade ductal carcinoma in situ (DCIS) are included in this category. Columnar Cell Lesions (CCLs) are characterised by distended acini lined by columnar epithelial cells with apical snouts. There may be associated epithelial hyperplasia (cellular stratification >2 cell layers but no complex architectural patterns). When CCL shows cytonuclear atypia, it is designated as flat epithelial atypia (FEA). FEA typically lacks well-developed architectural atypia (micropapillae, tufts, fronds, rigid bridges and punched out spaces: features of ADH /low-grade DCIS). In FEA, the nuclei are uniform, rounded and evenly spaced. CCLs are more frequent with tubular carcinoma (TC) and invasive cribriform carcinoma (ICC) (92 and 60 %, respectively) [5, 6, 12, 13]. Low-grade DCIS shows intraductal proliferation of evenly spaced, usually small monomorphic cells with hyperchromatic nuclei but inconspicuous nucleoli. Cells are arranged in cribriform and micropapillary patterns, with the cribriform pattern being predominant. The solid pattern is rare within this category of DCIS. Mitosis and necrosis are also infrequent. ADH is cytologically and architecturally similar to low-grade DCIS but its extent is limited (a 2–3 mm focal lesion confined to one or two duct spaces). ADH is a recognised risk indicator and a non-obligate precursor of low-grade DCIS and invasive BCs, though the risk of invasive carcinoma developing is smaller compared to DCIS [14].
Lobular neoplasia (LN), on the other hand, refers to non-invasive proliferative low-grade lesions consisting of a monomorphic population of generally small and discohesive cells that fill and expand the TDLU. LN encompasses both ALH and LCIS, which are morphologically similar but ALH represents an early/less well-developed lesion with partial involvement of acini. The morphological distinction of ALH and LCIS is however, somewhat arbitrary, depending on extent. There are rare variants of LCIS that show more aggressive features. These include pleomorphic and mass-forming necrotizing LCIS. LN can behave both as a high-risk lesion and a non-obligate precursor to BC [10, 11].
Molecular Features
Loss of heterozygosity (LOH) and comparative genomic hybridization (CGH) studies have established that low-grade and high-grade BCs are dissimilar at the DNA level. Low-grade precursors display a lower level of genomic instability with fewer chromosomal aberrations and exhibit recurrent losses of 16q and gains of 1q and 16p [2, 3], which often arise from an unbalanced chromosomal translocation involving chromosomes 1 and 16. Frequent loss of 16q has been demonstrated in all varieties of the low-grade neoplasia family viz. CCL [9], FEA [7], ADH [15] and low-grade DCIS [16]. The loss of 16q, which is a frequent (>70 %) event, involves the whole chromosomal arm in contrast to high-grade precursors where it is infrequent (<20 %) and occurs through a different process (LOH with mitotic recombination) [1–3, 17]. Although the loss of 16q is observed in both low-grade ductal and lobular precursors, the target genes differ in these two lesions. The gene involved in loss of 16q in LN is CDH1 (E-cadherin), which maps to 16q22.1. E-cadherin is now well characterised as a tumour suppressor gene in LN as evident by CDH1 mutation and loss of E-cadherin protein. Such mutations are rare in DCIS. Within the lobular neoplasia family, although the morphological boundary of ALH and LCIS is nebulous, evidence suggests that LCIS harbours greater copy number alterations [18] and possesses a higher risk of invasive BC development than ALH.
To characterise the LOH in DCIS, microsatellite-length polymorphisms at seven loci (AluVpa, ESR, D11S988, D13S267, D16S398, D17S1159 and D17S855) have been investigated from microdissected paraffin sections of DCIS cases [19]. Allelic loss or imbalance, reflecting LOH has been found to be commoner in invasive ductal carcinoma (IDC) rather than in DCIS. LOH in DCIS was most frequent at the D16S398 (26 %) locus. LOH at this locus was commoner in low- and intermediate-grade DCIS than in high-grade DCIS. Overall, microsatellite instability (MSI) at only one locus was more frequent in DCIS (28 %) than in IDC (6 %) (p < 0.001). The occurrence of MSI at multiple loci was similar in frequency in both DCIS (6 %) and in IDC (3 %). Together, these observations indicate that chromosomal losses of 16q may occur in low- and intermediate-grade DCIS and MSI involving multiple loci is uncommon in both IDC and DCIS.
Phenotypic Characteristics
More recent studies using cDNA expression array technology have confirmed that the core intrinsic molecular subgroups, including luminal, HER2 and basal, found in invasive breast cancer [20, 21] are replicated in DCIS, although at different frequencies [22] showing that the molecular heterogeneity of invasive carcinomas exists among in-situ lesions as well. Low-grade precursors are morphologically and immuno-phenotypically uniform (i.e. strongly positive for oestrogen receptor (ER) and luminal cytokeratins (CK) within lesional cells, but negative for basal CKs). Immunohistochemical studies usually demonstrate strong diffuse ER-positivity, PR-positivity and HER2-negativity within these lesions along-with low Ki67 labelling, and lack of expression of p53, p-cadherin and basal CKs (i.e. CK5/6 and CK14). Luminal CKs (CK19 and CK8/18), androgen receptor, Bcl-2 and cyclin D1 are often positive. In a recent study, [23] 112 cases of a series of 314 DCIS cases were classified as low grade. On phenotyping with routine ER, PR and HER2 staining, 71, 24, 9 and 8 cases distributed, respectively, to Luminal A, Luminal B, HER2 and triple negative subtypes. The overall % of low-grade DCIS within each of these phenotypic types was as follows: 52, 26, 18 and 20 %. This shows that the Luminal A subtype predominates in low-grade DCIS.
High Grade Precursor Lesions
Salient Histopathological Features
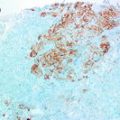
High-grade DCIS features (summarised from [10]) highly atypical cells within the duct space arranged in solid, cribrifrom or micropapillary patterns. The nuclei exhibit pleomorphism, are poorly polarised, often with irregular contour. Nucleoli are prominent and the chromatin is coarse and clumped. Mitotic figures, though common, are not a necessity for diagnosis. A frequently observed feature is comedo necrosis, where abundant necrotic debris in the lumina is surrounded by pleomorphic tumour cells. Necrotic intraluminal debris is often associated with amorphous microcalcifications. However, like mitoses, comedo necrosis is not obligatory for diagnosis. If typical morphological features are present even in a single space, this is deemed sufficient for diagnosis. High-grade DCIS may be diagnosed even if a single flat layer of highly atypical cells line a duct space, the ‘flat/clinging’ DCIS [7]. Cancerisation of lobule (involvement of the lobule by ductal epithelial cells) is more frequent with high-grade DCIS than with low-grade DCIS.
Molecular Features
High-grade DCIS is distinct from low-grade DCIS not only on morphology but also by phenotype and molecular genetics. They are usually aneuploid and rarely harbour the low-grade signature pattern (16q loss/1q gain) [24–27]. Whereas well-differentiated DCIS exhibits loss of 16q and 17p, high-grade tumours harbour significant losses of other allelic chromosomal arms including 1p, 1q, 6q, 9p, 11p, 11q, 13q and 17q as shown by array CGH studies [28]. In addition, high-grade DCIS displays gains at 17q, 11q and 13q [29]. Intermediate-grade DCIS shows features of both high- and low-grade DCIS, showing 16q loss but higher incidence of gains of 1q and losses of 11q in comparison to low-grade DCIS, but lacking the frequent amplifications at 17q12 and 11q13 that may occur in high-grade DCIS [16]. The average number of genetic imbalances in intermediate-grade DCIS though are higher than in low-grade DCIS. Flat type high-grade DCIS (clinging type DCIS) exhibits LOH at 11q, 16q and 17q in approximately 50, 60 and 40 %, respectively [7]. Other studies for LOH reveal that certain loci viz. D11S988 and D17S1159 are more frequently involved in high-grade DCIS. Especially, LOH at D11S988 was commoner in those cases with no evidence of comedo necrosis [19].
Certain specific genes have been identified to be amplified or inactivated in DCIS. cDNA micro-array technology has shown that the angio-associated migratory cell protein, a multifunctional protein with a putative role in motility and angiogenesis, is up-regulated in DCIS of high grade and in the presence of necrosis [30]. Another research group [31] identified upregulation of lactoferrin in DCIS, and downregulation of the oxytocin receptor and hevin (a cell adhesion related glycoprotein), though no correlation was identified with DCIS grade. HER2 is well recognised as being amplified in DCIS, with increased overexpression correlating with increasing nuclear grade [32] and will be further discussed in the next section on phenotype. Amplification of cyclin D1, a cell cycle regulator, has also been observed in DCIS [33] with overexpression being common in intermediate- and high-grade disease. Inactivating mutations of p53 are also observed in high-grade DCIS [32]. Another tumour suppressor gene apparently inactivated in DCIS is the IGF-II-receptor gene (on 6q) [33].
Phenotypic Features
The immunohistochemical profile of high-grade DCIS has been studied in various series. In a recent study of 314 cases DCIS [23], 202 cases were histologically of high grade. Phenotyping on the basis of ER, PR and HER2 staining, 63, 64, 44, 32 cases distributed respectively to Luminal A, Luminal B, HER2 and triple negative subtypes. The overall percentage of high-grade DCIS within each of these phenotypic classes was as follows: 48, 74, 88 and 80 %. In contrast to the low-grade DCIS of the series (discussed earlier), HER2 positives and triple negatives were more prevalent within high grades. Some studies [34] have investigated the phenotype of special histological variants of DCIS like cystic hyper-secretory DCIS. A special variant of DCIS with colloid-like luminal secretions, this subtype was found to be either intermediate or high grade and usually ER positive but HER2 negative and occasionally androgen receptor positive. Other studies have probed the distribution of intrinsic types of breast cancer in the context of whether DCIS shows associated invasion or not [23, 35–37]. In a study of 99 cases of pure DCIS and 96 cases of co-existing DCIS/IDC [36], there was a high rate of co-expression of CKs, ER-α, PR, HER2 and EGFR between DCIS and its co-existing IDC. The rate of discordance among biomarker expression was low and was present more commonly with high-grade DCIS/IDC. HER2, EGFR, CK5/6 and CK14 expression was associated with high-grade DCIS while ER and PR expression was observed low-grade cases. There was no difference in luminal CKs 8/18 expression between high- and low-grade categories. A recent immunohistochemistry based study [23] with 5 markers (ER, PR, HER2, CK5 and EGFR), revealed that for high-grade DCIS without accompanying invasive cancer, the distribution of phenotypic surrogates was as follows: luminal A (57.1 %), luminal B (11.9 %), HER2 (16.7 %), basal-like phenotype (0 %) and un-classified (14.3 %). For cases associated with invasive carcinoma, luminal cancers were also predominant viz. luminal A (58.2 %) and luminal B (12.7 %). HER2 positives were at a frequency of (7.6 %), but there were more basal-like (7.6 %) DCIS and in these cases the invasive component mirrored this phenotype. These results are in contrast to another study [37] which demonstrated differences in the occurrences of luminal A, luminal B, and HER2 phenotypes, but no difference in the basal-like phenotype with associated invasive malignancies. In another study [38] of 146 samples of DCIS and adjacent invasive malignancy, CK5/6 showed different distribution in DCIS and IDC, presenting a significant association with the triple negative phenotype in IDC, but a negative association within DCIS. A triple-positive profile (ER/PR/HER2 positive) and CK5/6 expression were negatively associated with invasion. In the low-grade DCIS subgroup, only CK5/6 expression exhibited a negative association with the probability of invasion. Given the variability especially in relation to the basal-like markers, further studies are warranted to establish the relationships between pure DCIS and co-existing DCIS/IDC.
Another area of substantial research interest has been HER2 expression and its significance in DCIS. In a series [39] of 103 pure DCIS and 38 cases of DCIS with <5 mm invasive carcinoma, pure high-grade, ER-negative DCIS with comedo necrosis showed a high frequency of HER2 overexpression. For DCIS with accompanying invasion, HER2 expression in the invasive component was higher than in DCIS. In a Chinese single institution study [40], 183 pure DCIS, and 43 patients of DCIS with invasion were studied where the HER2-positive subtype accounted for 27.9 % of the cases. Though on univariate analyses higher histological grade (Grades 2 and 3), and HER2 positive status were associated with invasion, on multivariate analysis only the HER2-positive status retained significance. In invasive cases, on further stratification of the accompanying DCIS as extensive or small (in relation to the total tumour area using a 25 % cut-off), HER2-positivity was associated with the cohort showing extensive DCIS. HER2 overexpression is also a typical feature of DCIS associated with Paget’s disease of the nipple.
In addition to the core findings above, the molecular biology of high-grade DCIS has been explored within BRCA mutation carriers [41]. DCIS in BRCA1 mutation carriers were high grade with high proliferation index and basal type by phenotype with low ER/PR/HER2 expression, but frequent CK5/6, CK14 and EGFR expression. On the other hand, within BRCA2 mutation carriers, DCIS exhibited the luminal phenotype in spite of being high grade. In BRCA1 and BRCA2 mutation carriers there was a high concordance between DCIS lesions and their concomitant invasive counterpart with regard to expression of individual markers as well as molecular subtype. The same research group has demonstrated that within both categories of BRCA mutation carriers, the hypoxia-related proteins HIF-1alpha, CAIX and Glut-1 are expressed in both DCIS and accompanying invasive cancer [42], and indicate the possible role of hypoxia in breast carcinogenesis and progression in these patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree