Biology
Depth
Patient
Ulceration
Positive deep margin
Excessive anxiety
Lymphovascular invasion
Regression
Age (younger)
Mitotic rate
Clark level IV or V
Vertical growth phase
>0.75 mm
Studies have reported an increased risk of nodal metastases in thin melanomas ≥0.75 mm when compared to <0.75 mm [8–10]. Han et al. [14] recently reported 6.3 % of melanomas ≥0.75 mm had positive lymph nodes, while only 2.5 % of melanomas <0.75 had positive lymph nodes. Other studies have reported rates of 5–15 % positive nodes for melanomas ≥0.75 mm, but 0–5 % for melanomas <0.75 mm [8–10, 12, 16–20] (Table 2). Positive nodes in melanomas <0.5 mm are even more unusual.
Table 2
Rates of SLN positivity in thin melanomas and correlating characteristics
Study | Breslow thickness | Rate of SLN positivity (%) | Comments/other factors correlating with + SLN |
|---|---|---|---|
Han et al. [8] | T1a < 0.76 | 0 | Mitotic rate > 1/mm2 (p < 0.05) Ulceration (p < 0.05) |
T1a ≥ 0.76 | 4.8 | ||
T1b < 0.76 | 18.2 | ||
T1b ≥ 0.76 | 12.5 | ||
T1 < 0.76 | 6 | ||
T1 ≥ 0.76 | 8.1 | ||
Murali et al. [10] | 0.51–0.74 mm | 3.8 | Lymphovascular invasion (p = 0.018) |
0.76–0.90 mm | 5.3 | ||
0.91–1.00 mm | 10.3 | ||
Wright et al. [16] | <0.25 mm | 8 | Age < 50 (p = 0.04) |
0.25–0.50 mm | 4 | ||
0.51–0.75 mm | 4 | ||
0.76–1.00 mm | 6 | ||
Ranieri et al. [17] | <0.75 mm | 2.3 | Mitotic index > 6/mm2 (p = 0.006) Clark level (p = 0.01) |
0.75–1.00 mm | 10.2 | ||
Wong et al. [18] | <0.75 mm | 0 | No other significant factors |
≤1 mm | 3.6 | ||
Hinz et al. [19] | <0.90 mm | 0 | No other significant factors |
0.90–1.00 mm | 4.1 |
Clark level has also been advocated by some as a predictor of nodal involvement in thin melanomas; however, the subjectivity of this classification has limited its utility. Ranges of SLN metastases in Clark level < IV are reported as 3.5–4.5 %, but this increases to 7.4–12.3 % in Clark level ≥ IV [14, 15, 17]. Additional studies have reported that Clark level is a predictor of disease when stratified by Breslow thickness to <0.75 and ≥0.75 mm [14]. Given this information, many continue to advocate for SLN biopsy with Clark IV tumors.
Although uncommon in thin melanomas, ulceration is a risk factor for more aggressive disease and secondarily, positive SLN. Yonick et al. [9] recently reported a five times increased risk in positive SLN in the presence of ulceration. Likewise, Han et al. [8, 14] reported ulceration increased the risk of a positive SLN. When stratified to Breslow thickness ≥0.75 mm, there was a 14.7% rate of nodal positivity in patients with ulceration, whereas only 6 % of patients without ulceration had a positive SLN [8].
Mitotic rate was included in the most recent iteration of the AJCC staging system, being used to discriminate stage IB patients from stage IA [3]. Although correlative for metastatic potential and an independent predictor of survival, the overall contribution of mitotic figures to lymph node positivity is yet to be clearly defined [15, 21–23]. Other studies have found a slight but nonstatistically significant increases in SLN positivity or a significance only among patients with lymph node positive disease [8, 10]. This is an area where further research is necessary.
Tumor regression refers to the tumor loss associated with inflammatory stromal changes around a melanoma [12]. The prognostic significance of this phenomenon is not entirely clear and conflicting data abounds, but it remains another factor that may be considered when deciding if a SLN biopsy is necessary. While some studies have advocated for a more aggressive approach to sentinel lymph node biopsy in the setting of thin melanoma and mitotic figures, others have found this unwarranted [14, 24–26]. Although currently used at some sites to promote SLN biopsy, regression is not currently a criteria according to the National Comprehensive Cancer Network guidelines nor was it suggested by the consensus guidelines published jointly by the SSO and ASCO in 2012 [27, 28].
Despite a litany of histologic features which make a thin melanoma “high risk” the risk of nodal involvement, even in patients with these features, is low. Importantly, the risk of the procedure and the cost remain modest, at worst, and the impact of identifying disease early is significant on outcomes. The cost of delaying intervention in patients with nodal metastases is likely considerable, as well [29]. Given these considerations, the comparative effectiveness of sentinel node biopsy in thin melanoma patients is a question with an ambiguous answer and unfortunately, preconceived biases and limited alternative approaches deter additional studies attempting to review this question.
4 Thick Melanomas and Sentinel Lymph Node
Thick melanomas are described as Breslow thickness ≥4 mm. These patients have a significant risk of regional metastases (60–70 %) but an equally high risk of systemic disease (70 %). The high risk of systemic disease in this population has therefore led many authors to question the utility of sentinel lymph node biopsy in patients with melanomas greater than 4 mm in depth rationalizing that their prognosis is more strongly linked to progression to stage IV illness than to lymph node status [30, 31]. Because the survival of these patients is poor overall, Balch et al. [32, 33] initially hypothesized that locoregional management via nodal dissection was unlikely to confer survival benefit. However, two recent studies advocated that sentinel lymph node status—even in patients with thick melanoma—was found to be an independent predictor of survival [30, 31]. Gershenwald et al. [31] looked at 116 patients with melanoma >4 mm thick and found that sentinel lymph node status was still the most powerful predictor of overall survival by univariate and multivariate analyses. Ferrone et al. [34] likewise looked at 126 patients with thick melanoma and found comparable rates of positive SLN (30 % vs. 39 %) and 3-year recurrence-free survival (76 % vs. 72 %). In the presence of conflicting data, many institutions routinely perform SLN biopsy even in those with thick primary tumors. With the advent of more robust therapeutic options for visceral disease and investigations demonstrating promising early results for genetic profiling of tumors, the role of surgical staging via sentinel lymph node biopsy becomes more ambiguous, generating a greater series of questions. The opportunity for early intervention in high-risk patients without the potential morbidity of surgical lymphadenectomy may pose a better alternative than the current paradigm in a subset of patients.
5 Overall Benefit of Sentinel Lymph Node Biopsy
The MSLT-1 trial has proven that early detection of regional metastases improves survival (Fig. 1); however, because the ability to select patients to undergo sentinel lymph node biopsy is impeded by the limitations of using histologic characteristics to determine biology, the procedure itself does not afford an overall survival benefit for all comers (intervention can only impact survival in the 17 % of patients who actually have nodal disease). This is compounded by the significant heterogeneity observed in the node positive group, which–with increasing recognition of microscopic and immunohistochemically detected disease, is likely to become more diverse. Survival in this group can range from 64 to 91 % depending on the population [35]. The resulting limitation of the sentinel lymph node procedure therefore, is that even in patients who are at the highest risk for having nodal metastases, nearly two-thirds will be undergoing a procedure that they do not need and, therefore, can derive no benefit. Within this context, the optimal improvement in this procedure will not be a technical one, but rather an intervention which aids in improving selection. Unfortunately, the ability to make this improvement will likely rely upon techniques other than histology such as genetic analyses or similar. Likewise, improvments in selection will be dependent on an ability to accrue large numbers of patients in order to discern even small differences in study groups. There is still much work to be done to define the group most likely to benefit from sentinel node biopsy.
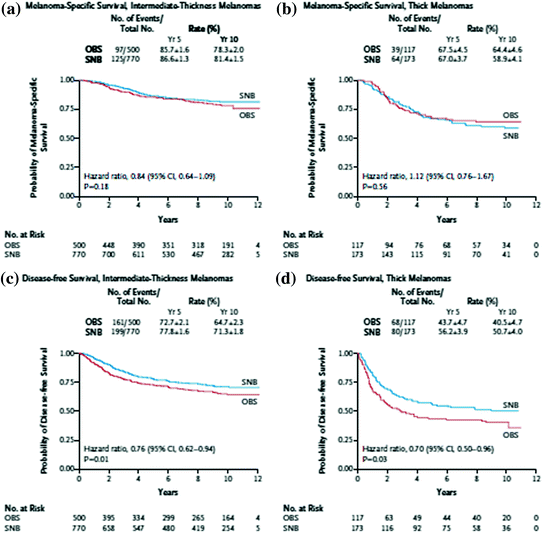

Fig. 1
Melanoma selective lymphadenectomy trial I (MSLT-1) results demonstrating both improved melanoma-specific survival and disease-free survival [29]. a Melanoma-specific survival, intermediate-thickness melanomas. b Melanoma-specific survival, thick melanomas. c Disease-free survival, intermediate-thickness melanomas. d Disease-free survival, thick melanomas
To work this direction, we could use large database studies, using propensity scoring to match those undergoing sentinel- lymph node biopsy with similar controls. While pooling of clinical data from multiple institutions has been done by the American Joint Committee on Cancer (AJCC), using a larger data set and standardizing the pathology variables (in which there was previously wide variability), could help us find an answer. Decision analyses could also assist in this process, and could effectively summarize the costs, benefits, and probabilities of each branch point. Though it may be difficult to assign accurate probabilities to some of the more qualitative outcomes, such as quality of life and patient satisfaction, application of the decision sciences could be very productive in defining the proper use of sentinel lymph node biopsies.
6 Completion Lymphadenectomy
The advent of sentinel node biopsy has made the management of nodal metastases even more controversial than the detection of nodal metastases. It is well recognized that in many diseases, management of regional disease is controversial (breast cancer, gastric cancer) and melanoma is similarly challenged. Using conventional histologic evaluation, only 20 % of patients undergoing CLND will have additional nodal disease, implying that as many as 80 % of SLN positive patients may be exposed to the risks of a second, more morbid procedure with potential long-term effects, without benefit. There are differences in the detection of metastatic deposits in sentinel nodes versus nodes in a completion lymphadenectomy specimen given the more rigorous examination with immunohistochemistry and serial sectioning applied to sentinel nodes. One could argue, that without the more intense scrutiny of the completion lymph nodes that is applied to sentinel nodes, the true incidence of nodal involvement in CLND specimens is not known. As a result, regional disease management after a positive sentinel lymph node remains a discussion point.
Although one might extrapolate from the MSLT trial that there may be survival benefit to clearing the nodal basin with completion lymphadenectomy (early detection of disease impacts outcome which would imply some potential benefit to regional disease control), other studies have not been so forthcoming. Outside of MSLT, there fails to be a proven survival advantage for undergoing a completion lymphadenectomy. Van der Ploeg et al. [36] recently evaluated 1,174 patients with sentinel node positive melanoma, 61 of whom did not undergo completion lymph node dissection. Completion lymphadenectomy did not show any influence on survival (HR 0.86, 0.46–1.61; P = 0.640). Another multicenter trial examined 134 SLN positive patients at 16 centers who did not undergo completion lymphadenectomy and compared them against a cohort of patients from Memorial Sloan Kettering Cancer Center who had a positive SLN and underwent completion lymphadenectomy [37]. There was no difference in nodal recurrence-free survival between the groups (P = 0.07) or in the disease-specific survival between the groups (P = 0.65). Other studies have documented similar results with no difference in recurrence-free or disease-specific survival [4, 38].
Other studies have attempted to delineate characteristics of the sentinel lymph node which may predict involvement of nonsentinel lymph nodes. Additional factors such as tumor burden, depth of invasion from capsule, microanatomic location, and maximum diameter of the largest tumor have all been considered as predictors of additional lymph node disease [4, 39–41]. Nagaraj recently published a metaanalysis to determine clinicopathologic variables most predictive of nonsentinel node metastases in the setting of a positive sentinel node [42]. There were nine factors including ulceration, satellitosis, neurotropism, >1 positive SLN, angiolymphatic invasion, extensive locations, macrometasases >2 mm, extranodal extension, and capsular involvement which predicted additional positive nodes beyond the sentinel lymph node. Unfortunately, this makes for a complex set of prognosticators when attempting to decide on completion lymphadenectomy and may not be as useful practically. Van der Ploeg et al. published a more straightforward study demonstrating that the burden of disease and allocation of tumor within the sentinel lymph node influences melanoma-specific survival. Patients with metastases <0.1 mm and found in the subcapsular area had a 5-year overall survival of 91 %. Nonsentinel lymph node rates for these patients was 2 %. The study concluded that completion lymph node dissection in these patients may be overtreating patients who have a survival that is equivalent to SLN negative patients [43].
The current guidelines of the 2008 National Comprehensive Cancer Network recommend either CLND or participation in a clinical trial for a positive sentinel lymph node. Despite these recommendations, only 50 % of patients in the National Cancer Data Base with a positive sentinel lymph node actually undergo CLND, clearly indicating some disconnect between recommendations and practice [44]. Reasons for lack of CLND in SLN positive patients are variable. Kingham et al. looked at over 2,000 patients who had undergone SLN biopsy, where 317 patients had positive SLN followed by lymphadenectomy and 42 patients with positive SLN did not. The patients not undergoing CLND were older (median age 70 vs. 56 years, p < 0.01) and had a trend toward thicker melanoma (Breslow 3.5 vs. 2.8 mm, p < 0.06). Additionally, as expected, there were a higher percentage of lower extremity melanomas in the group that did not undergo CLND (40 % vs. 13 %; <0.01) since many surgeons avoid groin dissections secondary to their high risk of complications and lymphedema. Bilimoria et al. and Cormier et al. [44, 45] found similar reasons for lower than expected rates of CLND such as older age, lower extremity melanoma, thin melanomas, and African-American race.
Perhaps most importantly, these studies, when analyzed in the context of the others, provide an ambiguity to the overall benefit to the patient undergoing completion lymphadenectomy. A survival benefit has not been proven, morbidity from many lymphadenectomies is high, and few patients in the sentinel node era actually recur in the nodal basin making palliative interventions a low priority. It is a challenge to demonstrate an overall comparative effectiveness to completion lymphadenectomy and it will be a long time before any data is available to provide any insight. Obstacles to our understanding this in greater detail include inherent bias toward the MSLT-2 trial (patients are only referred for possible observation if they are perceived as “lower risk”), low incidence of events in this population necessitating a large patient population with extended follow up, and finally a long “tail” in which events can occur before data is conclusively determined to represent a comprehensive review. With these obstacles, there will be a considerable delay before the questions surrounding lymphadenectomy can be answered.
Similar to the issues regarding the optimal use of sentinel lymph node biopsy, the uncertainty around completion lymph node dissections need to be explored using alternative research methods. Studies that make use of large databases and pooled multi-institutional clinical data will help us avoid the bias inherent in MSLT-2 and the long follow-up time required for meaningful results. Decision analyses can also help us examine how we should guide our patient through the process of choosing a completion lymphadenectomy or not.
7 Margins of Resection
7.1 1 versus 2 cm Margins
Until the 1970s, wide excision of all melanoma with 3–5 cm margins was the standard [46]. In the 1970s, there was recognition that different Breslow’s thickness and Clark’s levels may guide the need for a wider excision. In 1980s, the World Health Organization (WHO) melanoma group organized a randomized prospective clinical trial to determine optimal margin resection (1 cm vs. 3 cm) for thin melanoma <2 mm thick [47, 48]. There was no statistically significant local recurrence between the two margins. A follow-up study from 1998 confirmed an insignificantly higher (2.6 % vs. 0.98 %) risk of local recurrence in the narrow margin group with no difference in overall survival [46]. Meanwhile the Intergroup Melanoma Surgical Trial randomized 1–4 mm melanomas to 2 cm versus 4 cm excisions [49]. Neither the local recurrence (0.8 % vs. 1.7 %) nor the 5-year survival (79.5 % vs. 83.7 %) were statistically significant. A follow-up study in 2001 confirmed that neither 10-year local recurrence (2.1 % vs. 2.6 %) nor overall survival (70 % vs. 76 %) was statistically significant in the narrow excision or wide excision groups [50]. Finally, the United Kingdom Melanoma Study Group Trial found no difference in local recurrence (3.3 % vs. 2.8 %) or overall 5-year survival (68.2 % vs. 70 %) in 1 cm versus 3 cm resection margins in melanomas >2 mm [51]. When combining local and regional disease recurrence, however, there was a significant difference (37.1 % vs. 31 %; p = 0.05) between the groups. In overlapping these trials, the recommendations of a 1–2 cm wide local excision for a 1–2 mm melanoma were created, allowing clinicians the liberty of taking a 1 cm margin in cosmetically sensitive locations (Table 3).
Table 3
Randomized trials in primary melanoma excision margins
Trial | Melanoma thickness (mm) | Margins of resection | Local recurrence | Overall survival |
|---|---|---|---|---|
<2 | 1 cm versus 3 cm | 12 year | 12 year | |
1 cm—2.6 % | 1 cm—85.1 % | |||
3 cm—0.1 % | 3 cm—87.2 % | |||
p = 0.77 | ||||
1–4 | 2 cm versus 4 cm | 10 year | 10 year | |
2 cm—2.1 % | 2 cm—79 % | |||
5 cm—1 % | 5 cm—7.6 % | |||
p = 0.07 | ||||
Swedish melanoma study group [53] | 0.8–2 | 2 cm versus 5 cm | 10 year | 10 year |
2 cm—0.6 % | 2 cm—79 % | |||
5 cm—1.0 % | 5 cm—76 % | |||
French cooperative group [52] | <2.1 | 2 cm versus 5 cm | 10 year | 10 year |
2 cm—0.62 % | 2 cm—87 % | |||
5 cm—2.4 % | 5 cm—86 % | |||
p = 0.56 | ||||
United Kingdom melanoma study group [51] | >2 | 1 cm versus 3 cm | 5 year | 5 year |
1 cm—3.3 % | 1 cm—68.2 % | |||
3 cm—2.8 % | 3 cm—70 % | |||
p = 0.60 |
Two additional trials looked at even wider margins (Table 3). The French cooperative group randomized patients with thin or intermediate melanomas to 2 cm versus 5 cm local excision and found there was no difference in tumor recurrence, disease-free survival or overall survival for lesions <2 mm [52]. This was again confirmed in the Swedish Melanoma Study Group which looked at 2 cm versus 5 cm margins in melanoma ≤2.1 mm thick [53]. There was no difference in overall survival or disease-specific survival at 10 years.
No randomized trials have ever examined 1 cm versus 2 cm margins and, while an international trial has been written and proposed, accrual to this trial is considered to be an obstacle. This concern is largely based on the fact that most clinicians have a predisposition to use a 1 cm margin where anatomically or physically constrained. A single institution study recently validated these data [54]. Hudson et al. reviewed 2,118 patients with T2 melanoma who underwent 1 cm versus 2 cm wide local excision. With a median follow-up of 38 months, the local recurrence was 3.6 months in the 1 cm group and 0.9 % in the 2 cm margin group (p = 0.044); however, on multivariate analysis, this difference was no longer significant (p = 0.368). Overall 5-year survival, likewise, was not statistically significant (29.1 months vs. 43.7 months). This validated the current NCCN recommendations; however, given the biases and uncertainty of retrospective analyses, a randomized controlled trial is required to put this question to rest.
For lesions greater than 2 mm, there remained controversy over margins of excision. Thomas et al. [51] published the results of a multi-institutional randomized trial of 1 versus 3 cm surgical margins in melanoma >2 mm. In the 900 patient trial, a 1 cm margin was associated with a statistically significant risk of recurrence but no difference in overall survival. Unfortunately, this trial did not use sentinel lymph node biopsy, had a poor definition for what constituted “local recurrence,” and greater than 60 % of the recurrences were actually nodal in nature, which makes its modern applicability questionable. Still dissatisfied with the question of a 2 versus 4 cm resection margin for lesions >2 mm, Gillian et al. [55] published a trial specifically looking at these margins to determine overall survival. They found no difference in overall survival or in the risk of recurrence or death due to melanoma when using a 2 cm resection margin versus a 4 cm resection margin.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree