Trial
N
Histology (%)
CRT regimen (Gy)
Median survival
3 year OS (%)
p value
Lee et al. [20]
50 S
SqCC 100
Cisplatin, 5FU, 45.6
27.3 m
–
NS
51 CRT
28.2 m
Lv et al. [23]
80 S
SqCC 100
Paclitaxel, cisplastin, 40
36 m
51.3, 33.8 (5 year)
0.04 (CRT groups compared to S group at 5 years)
80 CRT (pre-op)
53 m
63.5, 43.5 (5 year)
78 CRT (post-op)
48 m
62.8, 42.3 (5 year)
Mariette et al. [89]a
98 S
SqCC 71
Cisplatin, 5FU, 45
43.8 m
48.6
NS
97 CRT
31.8 m
55.2
Walsh et al. [18]
55 S
AC 100
Cisplatin, 5FU, 40
11 m
6
0.01
58 CRT
16 m
32
Urba et al. [19]
50 S
AC 75
Cisplatin, 5FU, vinblastine, 45
17.6 m
16
NS
50 CRT
16.9 m
30
Burmeister et al. [21]
128 S
AC 62
Cisplatin, 5FU, 35
19 m
–
NS
128 CRT
22 m
–
Tepper et al. [22]
26 S
AC 75
Cisplatin, 5FU, 50.4
1.8 year
16 (5 year)
0.002
30 CRT
4.5 year
39 (5 year)
van Hagen et al. [25]
188 S
AC 75
Carboplatin, paclitaxel, 41.4
24 m
44
0.003
178 CRT
49.4 m
58
In summary, for patients with potentially resectable localized esophageal and GEJ cancers, several randomized trials as well as meta-analyses demonstrate improved survival and efficacy with preoperative chemoradiation therapy compared to local therapy alone (surgery or radiation).
5 Role of Adjuvant Chemoradiotherapy for Gastroesophageal Tumors
Several trials have evaluated the role of adjuvant chemoradiation in patients with resectable tumors of the GEJ or stomach. The INT 0116 trial investigated patients with AC of the GEJ or stomach and randomized 556 patients to surgery plus postoperative chemoradiation or surgery alone [27]. The adjuvant chemoradiation included 5-FU and 45 Gy. The median overall survival in the chemoradiation group was significantly improved at 36 months compared with 27 months in the surgery alone group. The survival benefit was confirmed in the 10-year follow-up study [28]. The study conducted by Lv et al. [23] mentioned above with only SqCC patients included a postoperative chemoradiation group which had a statistically improved survival compared with the surgery alone group although the study was not powered to detect differences between the pre-operative chemoradiation group and the post-operative chemoradiation group.
6 Role of Neoadjuvant Chemotherapy
Due to the controversy surrounding radiation therapy and its utility in esophageal and gastric cancers, multiple trials have evaluated chemotherapy prior to surgery compared to surgery alone. At least 9 randomized trials have evaluated this question (Table 2). Similar to neoadjuvant chemoradiation, these trials are a mixture of SqCC and EAC and the results are mixed regarding survival benefit. Six trials did not show a benefit [29–34] while four did show a significant survival benefit [35–38]. One of the largest randomized control trials was the MAGIC trial which randomly assigned patients with resectable AC (stage II or higher with no evidence of metastases) of the stomach (74 %), GEJ (11 %), or lower esophagus (15 %) to either perioperative chemotherapy and surgery (250 patients) or surgery alone (253 patients) [36]. Chemotherapy consisted of three cycles each pre- and post-operatively of epirubicin, cisplatin, and 5-FU. The complication rate and 30-day mortality of both groups was similar. The perioperative chemotherapy group had a statistically increased 5-year survival rate of 36 % compared to 23 % in the surgery alone group. One limitation of the treatment strategy is that only 42 % of the perioperative chemotherapy group actually received the postoperative chemotherapy. A similar trial by Ychou et al. [38] again included only patients with resectable AC of the stomach, GEJ, and distal esophagus and randomized 113 patients to the perioperative chemotherapy group and 111 patients to the surgery alone group. A key difference in this trial compared to the MAGIC trial was the patient population. In the MAGIC trial 74 % of the patients had gastric cancer compared to 25 % in this trial, and GEJ/distal esophageal comprised 26 % in the MAGIC trial compared to 75 % in this trial. The chemotherapy regimen in this trial included two or three cycles of cisplatin and 5FU preoperatively and three or four cycles postoperatively. The perioperative chemotherapy group had a significant increase in 5-year survival of 38 % compared to the surgery alone group at 24 %. Perioperative chemotherapy also significantly improved the curative resection rate from 73 to 84 %. Another large trial by the Medical Research Council (MRC) included 802 patients. This patient population included 67 % with EAC although it did not include gastric cancer [39]. Patients were randomized to preoperative chemotherapy consisting of cisplatin and 5FU followed by surgery or surgery alone. Overall survival was significantly improved in the preoperative chemotherapy group compared to surgery alone with a hazard ratio of 0.79. A follow-up study by Allum verified improved survival for the preoperative chemotherapy group with a 5-year survival of 23 % compared to 17 % for the surgery alone group [37]. This survival benefit held true for both EAC and SqCC. A similar trial by Kelsen did not show a survival benefit [33]. In this randomized trial 216 patients underwent preoperative chemotherapy with cisplatin and 5-FU and 227 patients underwent surgery alone. The histological type was split 50/50 between EAC and SqCC. There was not a significant difference in survival between the neoadjuvant chemotherapy group and the surgery alone group although there was a survival benefit for those patients that responded to chemotherapy. The reason that there was no difference in survival in this study is unclear as similar chemotherapeutic agents were used. One possibility for the difference was the study size of the MRC trial included almost twice as many patients and another is that the percentage of patients with SqCC versus EAC was different.
Table 2
Neoadjuvant chemotherapy versus surgery alone
Trial | N | Histology (%) | Chemotherapy regimen | Perioperative mortality (%) | 3 year OS (%) | p value |
|---|---|---|---|---|---|---|
Cunningham et al. [36] | 253 S | AC 100 | Epirubicin, cisplatin, 5FU (pre-op and post-op) | 5.9 | 23 (5 year) | 0.009 |
250 C | 5.6 | 36 (5 year) | ||||
Ychou et al. [38] | 111 S | AC 100 | Cisplatin, 5FU (pre-op and post-op) | 4.5 | 24 (5 year) | 0.02 |
113 C | 4.6 | 38 (5 year) | ||||
19 C | 12 | |||||
Schlag [30] | 24 S | SqCC 100 | Cisplatin, 5FU | 10 | 10 m (MS) | NS |
22 C | 19 | 10 m (MS) | ||||
Law et al. [32] | 73 S | SqCC 100 | Cisplatin, 5FU | 8.7 | 13 m (MS) | NS |
74 C | 8.3 | 16.8 m (MS) | ||||
Kelsen et al. [33] | 227 S | AC 53 | Cisplatin, 5FU | – | 26 | NS |
216 C | – | 23 | ||||
Ancona et al. [34] | 48 S | SqCC 100 | Cisplatin, 5FU | 4.2 | 22 (5 year) | NS |
48 C | 4.2 | 34 (5 year) | ||||
Allum et al. [37] (MRC) | 402 S | AC 67 | Cisplatin, 5FU | 10 | 17 (5 year) | 0.03 |
400 C | 10 | 23 (5 year) | ||||
Boonstra et al. [35] | 84 S | SqCC 100 | Cisplatin, etoposide | 4 | 17 (5 year) | 0.03 |
85 C | 5 | 26 (5 year) | ||||
Maipang et al. [31] | 22 S | SqCC 100 | Cisplatin, vinblastine, bleomycin | – | 36 | NS |
24 C | 17 | 31 |
7 Role of Adjuvant Chemotherapy
Adjuvant chemotherapy is generally only recommended in patients with positive lymph nodes. Studies that have included adjuvant chemotherapy after neoadjuvant chemotherapy and/or surgery include the MAGIC trial and the French trial [36, 38] although in both of these trials only about 50 % of patients intended to receive postoperative chemotherapy actually did. There are a few trials evaluating adjuvant chemotherapy only. Ando randomized 205 patients with esophageal SqCC to either surgery alone or surgery followed by cisplatin and vindesine. The study did not find a statistical significance in 5-year survival between the two groups [40]. A subsequent study by Ando again included patients with esophageal SqCC randomized to surgery alone versus chemotherapy including cisplatin and 5FU. The 5-year survival rates were 52 and 61 % for surgery alone and surgery plus chemotherapy, respectively. This difference was not statistically significant [41]. It is important to realize that both of these studies only included those patients with esophageal cancer and furthermore only SqCC histology.
The question of neoadjuvant chemotherapy compared to adjuvant chemotherapy has been evaluated by a Japanese trial in which patients with esophageal SqCC were randomized to cisplatin and 5FU either pre- or post-operatively. Overall 5-year survival rates were significantly improved in the preoperative chemotherapy group (55 %) compared to the postoperative chemotherapy group (43 %) [42].
8 Summary
In conclusion, the evidence suggests that neoadjuvant chemotherapy or chemoradiotherapy provides better outcomes compared to surgery alone for esophageal cancer, GEJ, and gastric cancers. Meta-analyses indicate a trend towards improved survival in neoadjuvant chemoradiotherapy compared to neoadjuvant chemotherapy. For those patients that have undergone resection for node-positive esophageal cancer without receiving neoadjuvant therapy, some form of adjuvant treatment is generally recommended although there is no evidence supporting chemoradiation versus chemotherapy alone. One of the barriers to using randomized control trials in CER is that to answer certain questions the number of patients needed to enroll would be prohibitive both logistically and financially. Utilization of large databases is often beneficial to examine these questions from a different angle. One important question that must be addressed is how are clinicians using the information from these trials and consensus guidelines to treat their everyday patients?
9 Implementation of Consensus Guidelines for Esophageal Cancer Treatment
It is evident that surgery alone is insufficient for treatment of locally advanced esophageal and GEJ cancers and a plethora of randomized trials indicate that neoadjuvant treatment is superior to surgery alone. Consensus groups such as the National Comprehensive Cancer Network (NCCN) recommend as standard of care neoadjuvant therapy for stage II and III esophageal cancer [43]. Multimodality therapy for esophageal cancer was advocated in the 1980s when it became evident that surgical resection alone resulted in poor outcomes. Neoadjuvant chemotherapy and chemoradiation was implemented into clinical practice after a few large randomized trials demonstrated survival benefits with neoadjuvant treatment in the late 1990s and early 2000s [18, 22]. The ideal treatment regimen including type of chemotherapy, use of radiation, and if so what dose, were largely unknown due to a heterogeneous and unstandardized mix of trials with often conflicting results. Because of this uncertainty Merkow evaluated the national trends for neoadjuvant use in esophageal cancer to determine the effect of these randomized clinical trials on current esophageal cancer treatment. The study evaluated 8,562 patients from the National Cancer Database (NCDB) that were surgically treated for esophageal cancer between 1998 and 2007 [44]. This study demonstrated that for stage I patients neoadjuvant therapy use significantly decreased from 23.5 % in 1998 to 11.2 % in 2007. For stage II and III patients neoadjuvant use significantly increased: from 48 % in 1998 to 72.5 % in 2007 for stage II patients and from 51 % in 1998 to 90 % in 2007 for stage III patients. Factors that were found to be associated with decreased use of neoadjuvant therapy for stage II and III patients were older age, severity of comorbidity, Medicare insurance coverage, clinical stage II disease, and residence in the western United States [44]. An additional factor evaluated using the NCDB was perioperative mortality. In this study evaluating over 1,000 different hospitals, there was no significant difference in perioperative mortality between the patients treated with neoadjuvant therapy compared to those undergoing surgery alone. There was a significant decrease in surgical margin positivity rate as well as lymph node positivity in those patients who underwent neoadjuvant treatment compared to the surgery alone group.
10 Implementation of Consensus Guidelines for Gastric Cancer Treatment
In a similar study to the one mentioned above, Sherman examined the implementation of gastric cancer guidelines into clinical practice using the NCDB [45]. In some clinical trials proximal gastric adenocarcinoma, GEJ, and distal esophageal tumors are treated similarly [27, 36, 38]. As mentioned before, the Cuningham (MAGIC), Macdonald (INT-0116), and Ychou trials demonstrated that adjuvant therapy use in gastric AC resulted in a significantly improved overall survival [27, 36, 38]. These trials were published in the early 2000s and it was unclear how the results of these trials translated into generalized clinical practice outside the auspices of a trial. Based on these studies, the NCCN guidelines recommend preoperative chemoradiation for localized GEJ AC and perioperative chemotherapy or postoperative chemoradiation therapy for localized gastric AC [43]. To determine the impact of the studies and guidelines, Sherman identified 30,448 patients from the NCDB who underwent surgical resection for a diagnosis of stage IB-III gastric adenocarcinoma between 1998 and 2007 [45]. The proportion of patients with stage IB-III gastric adenocarcinoma who received systemic therapy (either pre- or post-operatively) increased by 71 % (from 35.7 to 61 %) between 1998 and 2007 while the proportion of patients who underwent surgery alone significantly decreased. The largest annual increase occurred between 1999 and 2000 which coincides with the release of the INT-0116 trial findings. The use of neoadjuvant therapy was also significantly increased between 1998 and 2007 from 6 to 20 %, with the largest increase between 2005 and 2006 which corresponded with the release of the MAGIC trial results. Multivariate analysis identified several factors for predicting systemic therapy use (pre- or postoperative): young age, male, fewer comorbidities, higher income, and private insurance. The most predictive factor for receiving neoadjuvant therapy was tumor location in the gastric cardia.
11 Summary
These studies indicate that clinical treatment of gastroesophageal cancer in the United States is changing. These changes seem to correlate with the release of large randomized trials and consensus guideline updates. While many physicians are altering their treatment based on current literature and studies, many physicians have not yet implemented these changes.
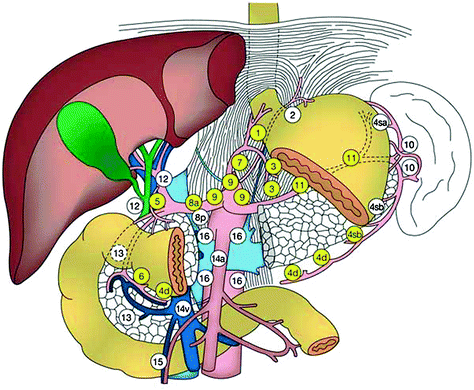
12 Gastric Cancer Lymph Node Dissection
One of the first clinicians to promote extended lymphadenectomies in gastric cancer was a Polish-Austrian surgeon Mikulicz [46]. He believed that aggressive locoregional control was paramount in controlling the orderly step-wise progression of cancer metastases through the lymph nodes. Even today the debate continues: those surgeons that advocate a D2 or D3 resection echo Mikulicz’s beliefs for locoregional control, and opponents argue that extensive surgery only adds perioperative morbidity and mortality without a survival advantage. Most patients who present with gastric cancer in the United States have advanced disease and the majority who undergo resection are found to have nodal disease [47, 48]. Controversy continues as Asian countries have been performing extended lymphadenectomies for decades while Western countries have only recently incorporated extended lymphadenectomies (D2) into their guidelines [43, 49]. Gastric cancer lymphatic drainage generally follows the vasculature. The most common locations for nodal metastases are lesser curvature (29 %), infra-pyloric (23 %), greater curvature (22 %), right cardia (19 %) and left gastric artery (19 %) [50]. Generally gastric lymph node dissections can be divided into D1 through D4 and the lymph node stations are numbered (Fig. 1). A D1 dissection involves removal of the stomach and the perigastric lymph nodes. In a D2 dissection, additional lymph nodes are removed including nodes along the left gastric, common hepatic, splenic, and left hepatoduodenal artery. D3 and D4 dissections include posterior hepatoduodenal and para-aortic lymph nodes [51]. Much of the controversy surrounding lymph node dissections in gastric cancer started in the 1980s when stage-specific 5-year survival in Japan was shown to be superior to that in the United States [52]. It was theorized that this difference was due to the extended lymphadenectomies performed in Japan compared to the United States. This stimulated multiple randomized trials comparing the extent of gastric lymphadenectomies.
One of the first trials was performed in South Africa by Dent in the late 1980s. In this study 22 patients were randomized to a D1 resection and 21 patients to the D2 resection group. While the morbidity was found to be higher in the D2 group, the survival at 3 years was similar between the two groups [53]. A larger trial was performed in the United Kingdom with a total of 400 patients who were randomized to a D1 or D2 lymphadenectomy [54]. For tumors in the middle and upper third, a distal pancreaticosplenectomy was performed to obtain the splenic hilar nodes and retropancreatic nodes. There was no significant difference in 5-year survival between the two groups: 35 % compared to 33 % for D1 versus D2 groups respectively. Although there was no overall survival difference, on multivariate analysis, those patients who underwent a D2 resection but did not undergo a distal pancreaticosplenectomy did have an improved survival rate compared to the D1 group. A similar trial performed in the Netherlands accrued patients with gastric cancer from 80 Dutch hospitals and randomized 380 patients to a D1 lymphadenectomy and 331 to a D2 lymphadenectomy [55]. The 5-year survival rates were not significantly different: 45 % for the D1 group and 47 % for the D2 group. There was a significant increase in complications (25 % vs. 43 %) and postoperative deaths (4 % vs. 10 %) in the D2 group compared to the D1 group. When the study was followed out to 11 years the survival for the two groups remained similar: 30 % versus 35 % for the D1 and D2 groups respectively [56]. When subgroups were analyzed, it was determined that a D2 lymphadenectomy may benefit those patients with N2 disease and that a pancreatectomy/splenectomy seemed to be the biggest risk factor of postoperative morbidity and mortality.
One of the main concerns regarding the two prior studies was a lack of surgeon training in D2 resections and variations between individual surgeons at different hospitals. Because of this the next trial performed in Italy included specialized surgeon training. In this study, 267 patients with gastric cancer were randomized to a D1 or D2 resection [57]. Unlike the previous studies, there was not a significant difference in morbidity in the D1 versus D2 groups (12 % vs. 18 %) or operative mortality (3 % vs. 2.2 % respectively) [58]. Similar to the previous studies, there was not a significant difference in 5-year survival between the D1 and D2 groups: 66.5 % versus 64.2 % respectively. When the subgroups were analyzed, it was found that patients with T2-T4 tumors and positive lymph nodes who underwent a D2 resection had a significantly improved 5-year survival rate compared to those in the D1 group: 59 % versus 38 %.
To determine the outcome of extended lymphadenectomies in Eastern patients, a randomized trial in Taiwan was performed at a single institution with 3 well-trained surgeons [59]. Patients were randomized to a D1 resection or a D3 resection. A D1 resection was defined as dissection of the perigastric lymph nodes in close proximity to the tumor along the greater and lesser curvatures [59]. A D3 dissection was defined as additional lymph node dissection around the blood vessels supplying the stomach such as the left gastric, common hepatic, and splenic, as well as lymph nodes in the hepatoduodenal ligament and retropancreatic region. Overall 5-year survival was significantly improved in the D3 resection group at 60 % compared with the D1 group at 53.6 %. Quality control for the surgeons was attempted by having only 3 surgeons perform the operations and each completed at least 25 D3 resections prior to the study. This study implies that in gastric cancer a D3 resection by well-trained surgeons offers a survival benefit compared to a D1 resection. A Japanese study evaluated whether an even more extensive lymphadenectomy known as a para-aortic lymph node dissection (PAND) was superior to the standard D2 lymphadenectomy with gastric resection [60]. The trial was performed in 24 hospitals and 523 patients with curable gastric cancer were randomized to either the standard D2 resection or a D2 resection plus PAND. There was a trend toward increased complications in the D2 plus PAND group with 28 % compared to the D2 group at 21 %. There was no significant difference in 5-year survival between the two groups: 70.3 % for the D2 plus PAND group compared to 69.2 % for the D2 alone group.
13 Gastric and Esophageal Lymph Node Examination in the United States
Regardless of one’s opinions about the ideal lymphadenectomy, what has been shown is that lymph node metastases are an important prognostic factor after gastric resection [61–64]. An adequate lymphadenectomy is necessary to allow for accurate pathologic staging. Several studies have investigated this issue and current consensus guidelines recommend a minimum of 15 lymph nodes to allow for reliable staging [43, 65–67]. Similar to esophageal and gastric cancer guidelines, it was unclear how treatment across the United States reflected these recommendations. Bilimoria evaluated how hospital type and volume effected the adequacy of the lymph node resection in gastric cancer [68]. The NCDB was used to identify 3,088 patients who underwent resection for gastric cancer. Of these patients, only 23.2 % had greater than or equal to 15 lymph nodes resected for pathologic evaluation, with an average of 7 lymph nodes [68]. The study also demonstrated that patients were significantly more likely to have greater than 15 lymph nodes examined if they underwent resection at an NCCN-NCI center compared to other academic or community hospitals. Patients were also significantly more likely to have greater than 15 lymph nodes examined if they underwent resection at the highest volume centers compared to high, moderate, or low volume centers.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree