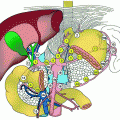
Fig. 1
Pancreas cancer research framework
1.1 Cancer Directed Care
Cancer directed care concerns issues of cancer detection, tumor factors, tumor targeted treatments, and subsequent cancer care.
Cancer detection involves topics such as screening, accurate diagnosis of pancreatic disease, and confirmation of malignancy. Research in this area includes identification of appropriate groups for screening, improvement of screening modalities, and development of innovative methods to detect malignancy in certain pancreatic lesions, such as small pancreatic lesions (<1 cm) or pancreatic cystic disease.
Tumor factors relate to the topics of tumor behavior and the interaction between the tumor and treatments. Research in this field includes defining tumor characteristics that have prognostic and predictive value. Identification of novel diagnostic biomarkers might improve our ability to predict outcomes and improve our ability to balance the harms and benefit of treatments. Discovery of markers that predict outcome response to certain agents will increase the efficiency of treatment delivery.
Research pertaining to tumor-directed therapy involves identification of targeted agents, their application such as the sequence of administration (neoadjuvant vs. adjuvant therapy), the optimal combination of treatments and the role and extent of surgery.
Subsequent cancer care relates to issues involving cancer surveillance and palliative care. Research within these topics involves developing methods to improve longitudinal tracking of patients, defining the optimal method and timing of cancer surveillance, developing treatments for late stage cancer symptoms, and improving measurement of relevant outcomes in the palliative setting [survival vs. quality of life (QOL)].
Of these topics in cancer directed care, six high priority research gaps have been identified and will be discussed in this chapter. The topics are as follows;
1.
Optimal chemotherapy regimens
2.
Value of radiotherapy
3.
Role of pre- versus peri- versus post-operative therapy
4.
Value of vascular and multi-visceral resection
5.
Resectable versus borderline resectable versus locally advanced unresectable tumors
6.
Management of intraductal papillary mucinous neoplasm (IPMN)
1.2 Pancreas Directed Care
Pancreas directed care involves issues regarding patient, surgeon and hospital factors and the surgical management of the pancreas.
Patient factors in pancreas care involve issues around patient selection and patient optimization. Research in this area involves identifying patient characteristics such as co-morbidities or frailty that predict outcomes and identifying interventions that lead to improved outcomes for patients after surgery.
Surgeon factors relates to issues involving adherence to established surgical standards, procedural volume, surgeon training, and experience. Similarly, hospital factors include hospital type, structural characteristics, culture and volume. Research in these areas involves determining how these factors relate to patient outcomes and how to improve the quality of care delivered to patients.
Surgical management of the pancreas includes issues such as operative technique and perioperative care. Research regarding operative techniques includes evaluation of new or emerging technology, technical challenges such as anatomic variations or involvement of vital structures, development of techniques to reduce surgical complications, and timing of surgery in the setting of other pancreas pathology (such as acute pancreatitis). Research pertaining to perioperative care includes issues such as pre-operative patient management, coordination of the surgical team, immediate post-operative care plan, and rapid surgical recovery strategies.
Of these topics in pancreas directed care, Three high priority research gaps have been identified and will be discussed in this chapter. The topics are as follows;
1.
Anastomotic technique and prevention of pancreatic fistula
2.
Use of peritoneal drains, octreotide, and prokinetic agents following pancreaticoduodenectomy
3.
Minimally invasive surgery versus open surgery
2 Research Gaps in Cancer Directed Care
2.1 Optimal Chemotherapy Regimens
Trials have demonstrated modest improvements in survival with the administration of adjuvant therapy, mostly consisting of 5-fluorouracil (5-FU) or gemcitabine, with or without radiation (Table 1). While the benefit of adjuvant therapy compared to surgery alone has been clearly demonstrated, the optimal regimen remains in question. Additionally, while new therapeutic agents such as FOLFIRINOX (leucovorin, 5-FU, irinotecan and oxaliplatin) and gemcitabine with nab-paclitaxel have proved effective in the metastatic setting [4, 5], their effectiveness in the adjuvant setting has, as of yet, not been determined.
Table 1
Summary of trials examining the benefit of adding adjuvant therapy
Year | N | Randomization arms | Overall survival (months) | p-value | |
|---|---|---|---|---|---|
ESPAC-1 [11] | 2004 | 289 | Chemotherapy | 20.6 | 0.009 |
No chemotherapy | 15.5 | ||||
Kosuge et al. [12] | 2006 | 88 | 5-FU + cisplatin | 15.8 | 0.904 |
Surgery alone | 12.5 | ||||
CONKO-001 [13] | 2007 | 368 | Gemcitabine | 22.1 | 0.06 |
Surgery alone | 20.2 | ||||
EORTC 40891 [14] | 2007 | 218 | 5-FU + XRT (40 Gy) | 15.6 | 0.165 |
Surgery alone | 12.0 | ||||
RTOG 9704 [15] | 2011 | 451 | Gemcitabine + 5FU/XRT (50.4 Gy) | 20.5 | 0.08 |
5-FU + 5FU/XRT (50.4 Gy) | 17.1 |
Randomized controlled trials (RCT) compare outcomes of participants randomly assigned to a new treatment group to those of participants assigned to a placebo or control group. They are regarded as the gold standard for evaluating new treatments and gaining regulatory approval due to their characteristic ability to address selection bias. However, modern oncology drug development faces increasing challenges. The average cost of bringing a drug to market is estimated to be $800 million [6]. Because adjuvant trials require the enrollment of a large number of patients and long-term follow-up, the actual therapies they assess may take 10–20 years to gain marketing approval [6]. Yet despite these astronomical time and monetary expenses, a report of the 2003–2010 phase III oncology drug trials found that more than 66 % of oncology drug trials fail to reach statistical significance in their primary end point [7]. This leads to an ever increasing backlog of potential anti-cancer drugs with promising results from early phase trials that have been untested.
In traditional clinical trials, initial parameters of study design such as sample size are pre-specified. This often leads to underpowered or overpowered studies due to inaccurate estimates of parameters. Re-thinking trial designs to include the CER-focused concepts may provide a method to enhance the operational efficiency, analytic efficiency and generalizability of RCTs. One such trial design is an adaptive clinical trial.
An adaptive clinical trial [8] accumulates evidence during an ongoing trial and actively reviews design elements and parameters to increase operational efficiency as well as the probability that trial participants actually benefit from participation. Examples of such “adaptions” include changing interventions or intervention doses, altering the rate of patient recruitment, or adjusting the probability of being randomly assigned to the different arms based on patient covariate information. Adaptive clinical trials allow new interventions to be added and less effective ones to be dropped without restarting the entire trial. In turn, this facilitates the comparison of therapeutic alternatives most relevant to current clinical practice and improves the timeliness and clinical relevance of clinical trials.
Adaptive clinical trials can be used for the rapid testing and development of new treatments for pancreatic cancer. Trials in pancreatic cancer often suffer from insufficient participation due to the limited number of patients with the disease. Aside from the tremendous costs required to conduct these trials, the scarcity of patients available for enrollment make it extremely difficult to employ a traditional clinical trial to compare all new anti-cancer drugs being developed at any given time as well as different dosing regimens, drug combinations, sequences of treatment and differential drug effects based on tumor profiles. An adaptive clinical trial design in pancreatic cancer could be protocoled to rapidly test new drugs combinations, doses and sequences. To expedite the fast turn-around for drug comparisons, an intermediary outcome that is predictive of clinical outcomes should be chosen as the end-point of the trial. In breast cancer, for example, since measuring overall survival after neoadjuvant takes a very long time, a more short-term marker such as clinical response (CR) was used in the I-SPY II trial [9, 10]. Similarly, in a highly aggressive tumor like pancreatic cancer, outcomes such as cancer recurrence or disease free survival (DFS) could be used due to the short amount of time needed to achieve these end-points. New drugs could be graduated if there were a high Bayesian predictive probability of achieving improved outcomes over the comparison group and dropped if a low probability of achieving improved outcomes was demonstrated. The graduated regimens would need to be re-tested and compared with other graduated drugs until the best drug is identified.
2.2 The Value of Radiotherapy
Patterns of recurrence in pancreatic cancer include both locoregional failure and systemic metastasis, with locoregional failure occurring in 50–60 % of cases [16, 17]. Even in cases where patients received adjuvant chemotherapy after surgery, locoregional recurrence rates remain as high as 30–60 % [11, 18]. The addition of adjuvant chemoradiation has been reported to decrease local recurrence rates to 20–40 % [19, 20]. However, there have been limited number of randomized clinical trials (Table 2) and the added benefit of combining radiation with chemotherapy in the adjuvant setting has yet to be determined. Of the trials that included radiation therapy, only ESPAC-1 [11] directly compared the addition of radiation to chemotherapy. ESPAC-1 used a 2 × 2 factorial designed to examine the benefit of chemotherapy over no chemotherapy and chemoradiotherapy over no chemoradiotherapy. The trial found that the clinical outcomes of patients who received chemoradiation were worse than those who did not. However, this trial has been criticized for the large number of patients who did not receive the intended therapy, its lack of standardized pathology review, and its high local recurrence rate [21, 22]. The debate about whether radiation should be included in the adjuvant setting is thus ongoing. A recently completed phase II trial, the EORTC 40013, investigated the role of chemoradiation over gemcitabine alone in the adjuvant setting [14]. While not designed to show difference, this study suggested that chemoradiation might improve outcomes over chemotherapy alone. To address this question, the ROTC 0848 was opened to accrural in November of 2009. This phase III trial aims to examine the role of both Erlotinib and chemoraditation as adjuvant treatment for patients with resected head of pancreas adenocarcinoma [23]. To meet its target accrual of 950 patients, this study involves 350 study locations, and is anticipated to close in year 2020.
Table 2
Summary of adjuvant trials examining the benefit of adding radiation
Year | N | Randomization arms | Overall survival (months) | p-value | |
|---|---|---|---|---|---|
1985 | 43 | 5-FU + XRT (40 Gy) | 20.0 | 0.03 | |
Surgery alone | 10.9 | ||||
ESPAC-1 [11] | 2004 | 541 | Chemoradiation | 15.9 | 0.05 |
No chemoradiation | 17.9 | ||||
EORTC 40891 [14] | 2007 | 218 | 5-FU + XRT (40 Gy) | 15.6 | 0.165 |
Surgery alone | 12.0 |
While the final results of these trials will shed some light on this question, RCTs are extremely costly and time consuming, and results often needs be confirmed through multiple sources before being accepted into practice. Adaptive clinical trials, as explained above, could also be used to provide evidence to address this question in an efficient and effective manner. Another method would be to perform an analysis of data collected either for this purpose in mind, or for other purposes, such as cancer registry data. Cancer registries collect information on patient demographics, cancer stage, tumor characteristics and outcomes [24, 25]. Some cancer registries such as Surveillance, Epidemiology and End Results Program (SEER) and National Cancer Data Base (NCDB) also collect information on first course of treatment that could potentially be used to compare treatment effects. This data can be used to compare patients who did and did not have radiation as a part of their first course of treatment. While these cancer registries collect information about whether patients did not or did not received specific categories of treatment, such as chemotherapy, radiation therapy or hormonal therapy, they typically lack details about the precise chemotherapeutic regimens that were used. To supplement these shortcomings, the NCDB has a mechanism to request further information from hospitals at an ad hoc basis (http://www.facs.org/cancer/publicncdb.html). All Commission on Cancer (CoC)-approved cancer programs are mandated to participate in these special studies. This ad hoc data could be used to collect detailed information on factors that could influence outcomes, such as type of chemotherapy used, surgical details (drains, pylorus saving, duct to mucosa, ductal stenting, post-operative octerotide use), and perioperative outcomes (surgical complications). Such data could be used in conjunction with existing data regarding patient and tumor characteristics and radiation treatment details. Data analysis, such as cohort studies, case-control studies and regression analysis, could be applied to this data to determine differences in outcomes while controlling for all known biases.
2.3 Pre- Versus Peri- Versus Post-operative Therapy
The ideal timing of additional therapy in relation to surgery continues to be debated. For borderline resectable pancreatic disease, there has been recent convergence of expert opinions towards a recommendation for neoadjuvant therapy. The Americas Hepato-Pancreato-Biliary Association (AHPBA)/Society of Surgical Oncology (SSO)/Society for Surgery of the Alimentary Tract (SSAT) Consensus Statement for combined modality treatment of pancreatic cancer [27] states “patients with borderline resectable pancreas cancer should be treated with neoadjuvant therapy, ideally in the context of a clinical trial.” For resectable pancreatic cancer no scientific evidence exists to determine the optimal sequencing of surgery in relation to the administration of systemic and/or radiation therapy.
The advantages of neoadjuvant therapy include early treatment of micrometastatic disease, and providing added time in which to identify patients with aggressive disease biology that will not benefit from surgery. Additionally, neoadjuvant therapy is a logical strategy to mitigate high rates of positive margins, while assuring that the patient will receive chemotherapy without the potential for post-operative delay or non-administration. Surgery following neoadjuvant therapy also provides tissue that can be compared with pre-treatment biopsy specimens, providing a valuable opportunity to study the direct tissue and molecular effects of therapy.
Unfortunately, it is difficult to examine the effects of neoadjuvant therapy using large databases. Except for some clinical trial databases or institutional databases, information regarding specific chemotherapeutic regimens is rarely collected even within cancer registry databases such as NCDB and SEER. Many patients receive chemotherapy in a facility that is different from the original treating hospital, making it difficult to obtain treatment data. Furthermore, there are often changes to treatment regimens during the course of treatment and inconsistent documentation by physicians administering chemotherapy.
On the other hand, information about radiation is often contained in large databases such as the NCDB and SEER. In the NCDB, start and end date of radiation, radiation treatment target (anatomic location, e.g. breast), radiation treatment modality (e.g. external beam, photons, etc.), regional dose (e.g. 50 Gy), boost treatment modality, and dosing information are collected and available to researchers for analysis.
While retrospective data analysis can provide supportive data, the benefit of neoadjuvant therapy for resectable pancreatic cancer will likely be best answered by well-conducted clinical trials. Fortunately, interest in the role of neoadjuvant therapies for pancreatic cancer has recently been growing. A query of ClinicalTrials.gov produced 43 trials examining the role of surgery plus some form of additional therapy for resectable and borderline resectable pancreatic cancers. Of these, 30 trials specifically examined the role of neoadjuvant therapy for resectable pancreatic cancer (Table 3). We anticipate that the maturation of these trials will provide further guidance in approaching these patients.
Table 3
Neoadjuvant therapy trials for resectable pancreatic cancer (queried May 2014)
Trial registration date | Patient eligibility criteria | ID | Status | Phase | Experimental treatment | Primary outcome |
|---|---|---|---|---|---|---|
Oct 2005 | Resectable | NCT00243854 | Completed | 1 | Neoadjuvant hypofractioned radiotherapy concurrently with weekly gemcitabine and an EGFR tyrosine-kinase inhibitor (OSI-774, Tarceva) | Side effects that will prevent surgery following this therapy |
Jan 2007 | Resectable and borderline resectable | NCT00426738 | Ongoing, but not recruiting | 2 | Neoadjuvant gemcitabine and oxaliplatin with radiation therapy | Two year disease free survival rate |
Feb 2007 | Resectable | NCT00438256 | Ongoing, but not recruiting | 1/2 | Neoadjuvant proton beam XRT+capecitabine (4 dosing arms) | Feasibility and tolerability |
Apr 2007 | Resectable or borderline resectable | NCT00456599 | Ongoing, but not recruiting | 2 | Neoadjuvant gemcitabine and oxaliplatin with radiation therapy | Two-year disease free survival |
Jun 2007 | Resectable | NCT00490360 | Completed | 2 | Neoadjuvant chemotherapy with gemcitabine /cisplatin | Resectability rate > 70 % after restaging |
Sep 2007 | “Radiographically resectable pancreatic cancer, as determined by a surgical oncology” | NCT00536874 | Ongoing, but not recruiting | 2 | Neoadjuvant gemcitabine and oxaliplatin | Overall survival at 18 months |
Nov 2007 | Resectable | NCT00557492 | Ongoing, but not recruiting | 2 | Neoadjuvant bevacizumab and gemcitabine in combination with sequential rapid fractionation radiotherapy | R0 resection rate and rate of complete pathologic response |
Feb 2008 | Resectable and borderline resectable | NCT00609336 | Ongoing, but not recruiting | 2 | Induction chemotherapy, neoadjuvant chemoradiotherapy, surgical resection and adjuvant chemotherapy. | Median overall survival of patients with adenocarcinoma of the pancreas |
May 2009 | Resectable | NCT00892242 | Ongoing, but not recruiting | 2 | Zoledronic acid | Safety and feasibility |
Dec 2009 | Resectable | NCT01027221 | Recruiting | 1/2 | Neoadjuvant photonradiation | Determination of an active local external beam radiotherapy dose leading to a maximal number of tumor infiltrating T-cells |
May 2010 | Resectable | NCT01130701 | Withdrawn prior to enrollment | 2 | Neoadjuvant capecitabine, panitumumab and radiation | 3 year progression-free survival |
Sep 2010 | Resectable | NCT01314027 | Currently recruiting | 3 | Neoadjuvant (gemcitabine/oxaliplatin) + adjuvant chemotherapy (gemcitabine) | Progression-free survival |
Feb 2011 | Resectable | NCT01298011 | Ongoing, but not recruiting | 2 | Gemcitabine and Nab-paclitaxel (Abraxane) | Grade III/IV histological response in tumor specimen rate after induction therapy |
Mar 2011 | Resectable or borderline resectable | NCT01333332 | Recruiting | 2 | Capecitabine, radiation | Safety/Efficacy study |
Jun 2011 | Resectable | NCT01372735 | Not yet open | 1/2 | Neoadjuvant short course IMRT | Local recurrence rate |
Jul 2011 | Resectable | NCT01389440 | Ongoing, but not recruiting | 2 | Neoadjuvant treatment with gemcitabine and erlotinib followed by Gemcitabine, Erlotinib and radiotherapy in patients with resectable pancreatic adenocarcinoma | Percentage of ancients undergoing with neoadjuvant chemoradiotherapy and R0 resection |
Jul 2011 | Resectable | NCT01494155 | Recruiting | 2 | Accelerated short course radiation therapy with proton beam capecitabine and hydroxychloroquine | Progression-free survival |
Aug 2011 | Resectable | NCT01419002 | Terminated due to low recruitment rate | 3 | Neoadjuvant RTx | Local recurrence free survival |
Sep 2011 | Resectable or borderline resectable | NCT01446458 | Recruiting | 1 | Modified FOLFIRINOX, SBRT | Maximum tolerated total dose of stereotactic body radiation |
Nov 2011 | Resectable and borderline resectable | NCT01470417 | Ongoing, but not recruiting | 2 | Neoadj nab-paclitaxel/gemcitabine + RT (in resectable patients) | Biochemical response rate, Radiographic response rate, pathologic downstaging and margin status |
Dec 2011 | Resectable | NCT01521702 | Currently recruiting | 3 | Neoadjuvant gemcitabine/oxaliplatin plus adjuvant gemcitabine | Progression-free survival |
Dec 2011 | Resectable or borderline resectable | NCT01726582 | Recruiting | 2 | “personalized” therapy | Resectability Rate |
May 2012 | Allpts | NCT01760252 | Recruiting | 2 | Capecitabine, oxaliplatin and irinotecan (CAPOXIRI) | Treatment adherence rate (TAR) |
Aug 2012 | Resectable | NCT01660711 | Recruiting | 2 | FOLFIRINOX chemotherapy | Percentage of patients able to complete the full course of preoperative chemotherapy and undergo a resection |
Sep 2012 | Resectable | NCT01783054 | Recruiting | 2 | Gemcitabine and abraxane. | Tumor Response |
Jun 2013 | Resectable | NCT01900327 | Not yet open | 3 | Neoadjuvant chemoradiotherapy (CRT) (Gemcitabine, external beam) followed by curative surgery + adj Gem | 3-Year survival rate |
Aug 2013 | Resectable | NCT01918644 | Recruiting | 1 | (SBRT, capecitabine, and surgery) | Incidence of dose-limiting toxicity |
Jan 2014 | Resectable | NCT02030860 | Recruiting | 2/3 | gemcitabine/abraxane with or without paricalcitol prior to surgery | Number of adverse events |
Jan 2014 | Resectable | NCT02047513 | Not yet open | 2 | Neoadj nab-paclitaxel/gemcitabine + surgery + adj nab-paclitaxel/gemcitabine | DFS |
Apr 2009 | Resectable | NCT00889187 | Terminated for excess toxicity | 1/2 | Capecitabine + accelerated short course radiation | Feasibility and tolerability |
2.4 Value of Vascular and Multi-Visceral Resection
The pancreas resides in close anatomic quarters with many vital structures. As a result, pancreatic tumors often involve structures other than the pancreas, such as major vessels and adjacent organs, which are traditionally regarded to signal aggressive tumor biology compromising the benefit of resection. However, some surgeons have challenged this paradigm, suggesting instead that involvement of these vital structures reflects the tumor’s precarious location rather than its aggressiveness.
Superior mesenteric vein (SMV) and portal vein (PV) resection and reconstruction during pancreaticoduodenectomy (PD) are generally considered to be reasonable surgical options for pancreatic cancers abutting or invading the SMV or PV. However, outcomes of these complex procedures remain controversial. Ramacciato et al. [28] and Chua and Saxena [29] found low operative mortality rates, ranging from 0 to 7 %, and 5-year survival rates around 12 %, suggesting that SMV-PV resection was a safe and feasible option that provided survival benefits similar to that of PD without venous involvement. Conversely, Worni et al. [30] using the National Inpatient Sample database found higher rates of intraoperative (OR 1.94, p = 0.001) and postoperative (OR 1.36, p = 0.008) complications than patients who underwent pancreatic resection alone. Likewise, Castleberry et al. [31] using the National Surgical Quality Improvement Program (NSQIP) database found that vascular reconstruction was associated with a doubling of post-operative mortality (5.7 % vs. 2.9 %, OR 2.1, p = 0.008) and increased morbidity (39.9 % vs. 33.3 %, OR 1.36, p = 0.02).
Arterial resections and multi-visceral resections for pancreatic cancers are similarly contentious surgical issues. Current guidelines define arterial involvement of the superior mesenteric artery, common hepatic artery and celiac artery as criteria of unresectability [32]. Yet as surgical resection remains the most effective therapeutic intervention for pancreatic cancer, the value of more radical resections continues to be debated. A systemic review and meta-analysis by Mollberg et al. [33] found simultaneous arterial resections to have higher perioperative mortality and lower 1-year survival than non-arterial resections, but more favorable survival compared to patients who did not undergo resection at all. Two recent studies of multi-visceral resection (MVR) revealed that patients undergoing MVR had a higher incidence of surgical morbidity but similar mortality and long-term survival compared to patients undergoing standard PD, and improved long-term outcomes compared to patients receiving palliative bypass (16 months vs. 6 months, p < 0.0001).
While the questions about the roles of PV and SMV, arterial and multi-visceral resections for pancreatic cancer may form three separate debates, all share a fundamental underlying question: Is involvement of these structures a sign of the tumor’s biological aggressiveness or is it simply a bystander result of the tumor’s close proximity to these structures?
Attempting to answer to this question is difficult because two different related issues need to be examined concurrently. First, we must address whether a tumor’s involvement of vascular or visceral structures has an effect on long-term prognosis. Second, we must understand whether the addition of vascular or visceral resection leads to worse outcomes (Table 4). Because retrospective analyses have typically compared patients with vascular involvement who have undergone radical surgery (A) to patients without vascular involvement who did not undergo radical surgery (D), the validity of the comparison is questionable. Ideally, we would be able to examine the outcomes between patients with or without vessel or visceral involvement among patients who underwent extensive surgery (A vs. B), or among those who did not undergo extensive surgery (C vs. D). However, performing extended surgery on a patient without vascular involvement (B) or not doing surgery on a patient who has vascular involvement (C) would not be deemed ethical in a clinical trial setting and therefore cannot be compared in this way.
Table 4




2 × 2 matrix breakdown of the issues involved in determining if vessel or visceral involvement leads to worst outcomes
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree