Fig. 1
Non-disease factors that influence receipt of lung cancer treatment
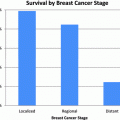
Limited disease is treated with chemotherapy and radiation therapy, while extensive disease is often treated with chemotherapy. Five-year survival rates for limited and extensive disease are poor (10–13 % vs. 1–2 %) with the median time of survival 15–20 months and 8–13 months respectively [8–10].
The remainder of this chapter will be focused on CER for NSCLC, where surgical intervention plays a role in early stage disease.
NSCLC accounts for the majority (approximately 85 %) of lung cancers. Localized disease is where the cancer is limited to one lobe of the lung, and does not involve the mediastinum. Stage I disease (small lesion, without involvement of any lymph nodes) and Stage II disease (larger lesion without lymph node involvement, involvement of structures that can be resected such as the chest wall or diaphragm, or involvement of hilar lymph nodes) makes up about a third of patients with NSCLC [11]. Although validation hasn’t occurred with randomized clinical trials, good results and long-term survival data have established surgery as the treatment of choice for localized disease in those patients who are medically operable [12]. Surgical resection has also found a role for select patients with stage IIIA disease as well (involvement of ipsilateral mediastinal nodes).
2 Surgeon Factors
2.1 Extent of Resection
The surgical resection of a single lobe of the lung, or lobectomy, is the procedure of choice for early stage NSCLC. The lung cancer study group [13] conducted a prospective randomized clinical trial comparing limited resection (segmentectomy or wedge resection) versus lobectomy in the management of early stage (less than 3 cm, and absence of lymph node involvement) NSCLC. When the group first reported their results, of the 247 patients followed for a minimum of 4.5 years, patients undergoing limited resection had an observed 75 % increase in recurrence rates, 30 % increase in overall death rate, and an observed 50 % increase in death with cancer rate compared to patients undergoing lobectomy. Interestingly, errors in accounting for patients lost to follow-up were noted by Dr. Frank Lederle, and detailed in his letter to the journal [14]. This prompted a second review of the study that uncovered 12 additional recurrences and 3 additional deaths. Using the corrected data, there remained a survival benefit to the lobectomy group (5-year survival 73 % vs. 56 %), but a decrease in the rate of recurrence (5-year 63 % vs. 78 %). The rate of distant recurrences was the same in both groups, whereas limited resection patients experienced threefold higher rate of locoregional recurrence (5.4 % vs. 1.9 %). In the new multivariate analysis, weight loss replaced performance status as a significant predictor of overall survival. Though the numbers, graphs and multivariate findings changed slightly, the overall conclusions of the lung cancer study group study were not altered by the corrected data [15].
Lobectomy became the norm for those patients with adequate pulmonary reserve. Additional observational studies demonstrated improved survival in individuals with earlier stage disease who undergo resection (lobectomy) [16–18]. Most surgical series demonstrated five-year survival for stage I NSCLC in the range from 55 to 72 % [19, 20], with even more favorable results reported for those with small (less than 3 cm) peripheral lesions [21, 22]. In contrast, the 5-year survival of patients with Stage I lung cancer not treated surgically is reported to be from 4 to 14 % [23–25]. The role of surgery for Stage IIIA disease is limited. If previously unsuspected microscopic disease is found in the mediastinal nodes at the time of resection, then proceeding with lobectomy followed by adjuvant therapy is recommended [26]. In those patients where the mediastinal nodes are prospectively identified with sampling and imaging, then multimodality treatment is recommended, with concurrent chemotherapy and radiation therapy. Surgical resection can subsequently be an option in a subset of these patients after chemoradiation with low volume or microscopic mediastinal nodal disease involvement, where resection is technically feasible [26].
Proximal tumors with endobronchial involvement traditionally were treated with pneumonectomies. In the modern era, sleeve resection of the involved airway with lobectomy is preferred over pneumonectomy as a result of similar oncologic results, better preservation of pulmonary function, and avoidance of the complications associated with pneumonectomy [27].
There has been a renewed interest in the role of sublobar (limited) resection. Sublobar resection may be performed either as the removal of one or more anatomical segments (segmentectomy) or as a non-anatomical wedge resection. Sublobar resections can be an option for those patients with early stage disease who cannot tolerate a lobectomy due to decreased pulmonary reserve, or medical co-morbidities. Several prospective studies have reported favorable outcomes in those patients undergoing segmentectomies or wedge resections for peripheral <2 cm in size, N0 lung cancers [28–30]. There are two ongoing clinical trials focusing on this issue. The Cancer and Leukemia Group B (CALGB) trial 140503 [31] has had a difficult time accruing patients since its launch in 2007, and aims to directly compare lobectomy versus limited resection among patients with peripheral tumors measuring <2 cm in size. In Japan, the nearly accrued JCOG 0802/WJOG 4607 L 1,100 trial is comparing the outcomes of peripheral invasive adenocarcinomas of less than or equal to 2 cm in size treated by lobectomy or segmentectomy. Another Japanese study (JCOG 0804/WJOG 4507L) has completed accrual evaluating the role of limited resections in the management of non-invasive adenocarcinomas. Results from these Japanese studies are awaited [32, 33]. Even after findings from these studies are released, CER opportunities will be present (Table 1).
Table 1
Surgeon factors—extent of resection
Dimension of care | Opportunities for CER study |
Extent of lung resection for early stage lung cancer | • Can the findings from recent randomized clinical trials on lobectomy versus sublobar resection be replicated in real-world settings? |
• What is the cost-effectiveness of performing segmentectomies (which is not a direct focus of training in today’s environment and thus require a learning curve) if indeed it is proven to be equivalent in outcomes to lobectomy? | |
• What are the quality-of-life implications for patients with respect to lobectomy, segmentectomy, and wedge resections? Should quality of life be the driving factor in decisions in how much lung to resect in contrast to cancer survival times, and for which patient populations is this relevant? |
2.2 Video-Assisted Thoracoscopic Surgery (VATS) Versus Open Lobectomy
VATS is a minimally invasive thoracic surgical procedure that can be utilized for the diagnosis and treatment of intra-thoracic diseases. Many procedures that historically were performed with an open thoracotomy are now performed as VATS. Absolute contraindications for VATS are the same as for a thoracotomy including the inability to perform a complete (R0) resection with suitable residual cardiopulmonary reserve, lymph node metastasis beyond regional lymph nodes, and widely metastatic disease [34, 35].
VATS procedures use ports that for the most part are <2 cm in length, except for one access or utility incision that ranges from 4 to 8 cm in length through which instruments can be inserted and allow for removal of the resected lung at the completion of the case. The important principle that is maintained during a VATS resection procedure is that a rib-spreader is not used. By avoiding muscle splitting, and rib-spreading with a retractor, VATS is believed to result in less pain, earlier ambulation, and fewer postoperative complications [36]. VATS lobectomy was first performed in 1992, and has been rapidly adopted in the surgical management of lung cancer. Several studies have shown lower rates of postoperative complications associated with VATS when compared with open lobectomy for early stage lung cancer [37–41]. Further studies have shown improved functional outcomes and equivalent oncologic efficacy as well [42–45].
Interestingly, cost effectiveness analyses have shown variable results. A study using the Surveillance, Epidemiology, and End-Results Medicare database found that VATS lobectomy was associated with a shorter length of stay (LOS) but not with differences in costs [36]. One explanation offered in the study was that payer reimbursement was linked to episodes of care rather than LOS. Other studies using the all-payer Nationwide Inpatient Sample [44, 45] described lower complication rates and LOS for VATS lobectomy, but no differences in costs. In contrast, an all-payer Premier Perspective database study [41] found that adjusted inpatients costs were $700 lower for VATS. A study from our group using the MarketScan database examined whether VATS lobectomy was associated with lower 90-day costs—thus assessing for costs and complications beyond the index hospitalization [46]. We found that the biggest driver of cost was prolonged LOS (PLOS) at the index hospitalization, VATS lobectomy was associated with lower rates of PLOS, and that the cost difference of the VATS approach extends only minimally into the period after discharge (i.e., health care use after discharge was only minimally different between the VATS and open thoracotomy groups). Outpatient use and readmissions accounted for approximately 16 % of the total 90-day costs of care after lobectomy. Emerging evidence suggests that the pressure to discharge patients earlier after lung resection may be driving readmission rates, independent of the surgical approach [47, 48].
Although use of VATS has increased over time, open thoracotomy is still the most widely used procedure for lobectomy with less than 50 % of lobectomies in the US performed using VATS [49]. Factors that have been proposed to explain this finding include insufficient training or experience, difficulty to achieve competency with minimally invasive approaches in low volume centers, and a belief that there remains an insufficient level of evidence for safety and efficacy. However, there are now two large institutional prospective cohort studies involving 1,100 and 500 patients respectively [50, 51], a randomized clinical trial [52] and two meta-analyses [53, 54] that have demonstrated safety and efficacy of the approach. The Cancer and Leukemia Group B (CALGB) 39802 trial [55] was a novel multi-institutional safety and feasibility study that not only standardized the definition of VATS lobectomy, but also standardized surgeon credentialing. The rigorous credentialing process had participating surgeons attend a course to review technique; submission of an unedited video tape, operative and pathology reports from a VATS lobectomy for central review; and participation in an animal laboratory. Surgeons were required to perform at least five VATS lobectomies before being credentialed. Eleven surgeons at six centers underwent credentialing, and the subsequent success rate, morbidity and mortality observed in the study achieved or surpassed prior levels cited in the literature. The study demonstrated a method for surgeons who had no prior experience with the technique to attain sufficient training and expertise in a supervised real-world environment.
Robotic-assisted thoracoscopic surgery (RATS) approaches for lobectomy have recently emerged as an alternative to traditional VATS. Initial results of robotic lobectomy have shown the same benefits achieved with VATS approaches as compared to open thoracotomy can be maintained [56–58]. Additionally, advocates for RATS cite benefits of improved ergonomics, three-dimensional optics, and wristed instrument motions. On the other hand, opponents of robotic surgery have cited increased costs and longer procedure times [59]. It is still to early to tell whether RATS is truly an advance in surgical technique, or as nay-sayers are fond of stating—a marketing gimmick. Cost analyses can be challenging for robotic programs as the cost of the robotic platform varies between institutions (purchase of a new robot versus incorporation of RATS into a facility with an existing platform), and that theoretic costs include the price of the robotic system divided by the total number of robotic cases (all specialties) performed. However, this shouldn’t deter us from assessing the impact of RATS into practice, as similar issues are raised when assessing the introduction of any new surgical technology. Even if one is not convinced that RATS is a significant advance as compared to VATS lobectomy, there may be other reasons for surgeons to transition to robotic surgery, including the performance of other thoracic surgical procedures such as a thymectomy, robotic technology will continue to evolve, and more clearly delineated benefits of robotic surgery may become the reality in the future. What is missing till date is a standardized detailed credentialing process for RATS similar to that which was outlined in CALGB 39802. CER opportunities with respect to minimally invasive approaches are outlined in Table 2.
Table 2
Surgeon factors—type of surgical approach
Dimension of care | Opportunities for CER study |
Minimally invasive lung surgery | • If PLOS at index hospitalization is the main driver of cost difference, are there evidence-based strategies to mitigate the frequency of PLOS that can be incorporated into systematic practice? Can these strategies be found from programs that incorporate VATS approaches as part of their practice? |
• VATS lobectomies are more commonly performed by high-volume surgeons working at high-volume or teaching hospitals. Are the lower 90-day costs only associated with VATS (i.e. more attributable to environment of practice) or directly attributable to VATS? | |
• Can a standardized credentialing process be implemented for Robotic VATS lobectomy, accounting for feasibility, safety, efficacy and cost-effectiveness? | |
• Can a prospective, pragmatic multi-institutional study be performed comparing robotic VATS, traditional VATS and open thoracotomy approaches for lobectomy for short-term outcomes, oncologic efficacy, and cost-effectiveness? |
2.3 Specialty Training
The issue of who should provide care has led to considerable debate about access, and healthcare disparities. Surgeon specialty has been shown to be associated with better post-operative outcomes among high-risk operations [60–62]. In the United States, the majority of lung resections are performed by general surgeons [63]. However, there have been several studies demonstrating that board-certified thoracic surgeons have lower rates of operative mortality with lung resections compared to general surgeons [64–66]. In a study conducted by our group in the SEER-Medicare population [67], we extended the analysis to compare the results of board-certified general thoracic surgeons (GTS), board-certified cardiothoracic surgeons (CTS) (those who performed both cardiac and thoracic procedures as part of their practice), and those treated by general surgeons (GS). After adjustment for several well-known prognostic factors for survival, patients under the care of GTS had an 11 % lower risk of death compared with those treated by GS. General thoracic surgeons used preoperative and intraoperative staging procedures more often than GS or CTS, more often applied video-assisted thoracoscopic techniques, and less often performed bi-lobectomy (precision of resection).
Common themes in these studies were influence of provider volume on the overall effect, as well as more consistent process-of-care measures by specialty surgeons. As there is a trend towards increasing specialization amongst surgeons, other factors that may have influenced decision-making include training in the modern era, with inclusion of evidence-based protocols, and multi-disciplinary participation in tumor boards amongst specialists. The results however of all these studies are relevant to the overriding issue of how best to improve the quality of thoracic surgical care. One option might be to encourage referral of potentially resectable lung cancer patients to GTS. This of course would need to be accompanied by the addressing of issues such as barriers to access, especially amongst low-income patients and those that live in rural areas, as well as workforce issues. Attempting to improve outcomes by selective referral without addressing infrastructure and workforce issues could lead to vast segments of the population without access to surgical resection for an otherwise uniformly fatal disease. Opportunities for CER are detailed in Table 3.
Table 3
Surgeon factors—specialist care
Dimension of care | Opportunities for CER study |
Surgeon specialty (general thoracic surgery [GTS] vs. cardiothoracic surgery [CTS] vs. general surgery [GS]) | • What are the workforce implications if a policy was created to selectively refer patients with early-stage lung cancer to only board-certified GTS? For both GTS and CTS? |
• If the workforce implications were not practical, how can CTS or GS predominantly serving an underserved population gain additional expertise to mitigate against some of the factors leading to disparity in outcomes? | |
• Should there be a central credentialing process for those surgeons who wish to perform surgical resections for lung cancer? |
3 Patient Factors
As overall outcomes for lung cancer treatment are poor, opportunities exist to explore the impact of treatment choices on the patient. When one traditionally thinks of patient factors, the focus has been on co-morbidities. In surgery, the decision-making process is often situational. Patient autonomy and participation can be influenced by medical condition, surgeon factors, patient educational level, and availability of evidence-based information on the particular condition. The degree of decisional authority assumed by patients can lead to three types of surgeon-patient relationships—the surgeon as agent, shared decision-making, and informed decision-making. Large-scale studies on decision-making have not been performed till date for lung cancer, but are anticipated to increase in the years ahead alongside the patient-centered movement.
The surgeon as agent occurs when the surgeon acts as an expert adviser who incorporates the values of the patient when making a treatment recommendation. In this model, the patient role is passive, as the surgeon assumes the values of the patient, and has total command over the decision-making process. Patients may be subjected to biased treatment if the surgeon only gives or delivers limited treatment options. In contrast, the informed decision-making model is one where the surgeon is recognized as the one who has technical expertise, but the patient plays an active role eliciting and understanding information about their treatment choices. The surgeon in this model doesn’t volunteer their opinions, but rather presents the patient with various treatment options, allowing the patients to arrive at their own conclusions. In between these two extremes is shared decision-making. Here the surgeon and patient are equal partners, where each freely exchanges information and preferences about treatment options to arrive at a mutually acceptable decision. This works especially in those situations where there is ambiguity in treatment of choice, and helps to align the decision-making with patient’s preferences and values.
Though surgeons may profess to always include patient-centered values in their discussions, there are plenty of opportunities for improvement. Some of these issues and opportunities for CER are detailed in Table 4.
Table 4
Patient factors
Dimension of care | Patient perspective | Opportunities for study |
Determination of patient’s values, preferences and expressed needs | • Short-term and long-term goals of care | Can identification of health beliefs, practices and specific ethnic and cultural groups lead to better decision-making for the lung cancer patient population? |
• Level of involvement in decision making (surgeon as agent, shared decision-making, informed decision-making) | ||
• Expectations of clinic visit and healthcare team | Can clinical interview protocols elicit patients’ perceptions about their illness and their expectations of treatment? | |
Coordination of care and integration of services within a clinical setting | Coordination of delivery of care by a multidisciplinary team | Does the patient get consistent information from different clinicians? |
Do dedicated patient navigators have an impact on the delivery of care for lung cancer? | ||
How can one improve efficiencies in the diagnostic and staging work-up of a potentially resectable lung cancer patient? | ||
Are there evidence-based interventions that can be performed to optimize patients’ health prior to elective surgical intervention? | ||
Can a set of processes of care be delivered as a guaranteed value bundle for each and every patient, all the time? | ||
Communication between patient and providers | Dissemination of accurate, timely and appropriate information | Are treatment decisions aligned with patient-preferences and values at each point of contact with the health system? |
Education about the long-term implications of disease and illness | Will electronic medical records, and open access to records for patients lead to better communication for members of the healthcare team, referring providers and patients? | |
Cancer survivorship plans | Transition and continuity from one locus of care to another | What is the best method for delivery of a cancer survivorship plan for patients, family members and primary care providers that outline treatment summary, surveillance plans, any ongoing treatments, and health risks that are now in play as a result of past treatment? |
4 Healthcare System Factors Related to Clinical Decision Making
4.1 Impact of Practice Environment
In principle, the Patient Protection and Affordable Care Act (ACA) signed into law in March 2010 seeks to improve health care delivery. The goal of ACA is to create a movement of payment reforms, in which private insurance companies would follow the lead of successful government payment reforms, such as bundled payments, and ultimately create system-wide changes for reimbursement [68]. Changing the reimbursement structure for providers will inevitably create new issues for surgeons who are making decisions for their patients. Payment reforms began in 2011 and 2012, and will continue through 2016. Two programs designed to restructure the way health care is delivered have been proposed under ACA, namely Patient-Centered Medical Homes (PCMHs) and Accountable Care Organizations (ACOs). These programs are designed to improve care coordination by encouraging use of electronic medical records, changing providers’ financial incentives by including quality measures in reimbursement, and ultimately moving away from a fee-for-service to one where quality of care is valued [69].
The ACO movement has led to increased consolidation and integration in the medical marketplace. Hospitals are buying practices to keep their market share intact and to have access to electronic record systems and other infrastructure that are expensive to capitalize. Awareness emerges for surgeons that their medical decisions can potentially negatively influence their income. This is not necessarily unethical, as cost containment has been recognized as an important circumstance in good decision-making [70]. There will be the introduction penalties for lack of delivery of quality care, which could affect physician reimbursement. Adoption of rigid guidelines for the treatment of patients is anticipated in years to come, as well as expansion of care plans. All of these are attempts at decreasing variation in care, decreasing length of stay, and reducing use of resources.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree