Figure 6.2 Adjusted mean (95% CI) percent change from baseline in body weight after 12 weeks of dapagliflozin treatment. Reproduced from List et al. (2009) with permission from the American Diabetes Association.©2009 American Diabetes Association.
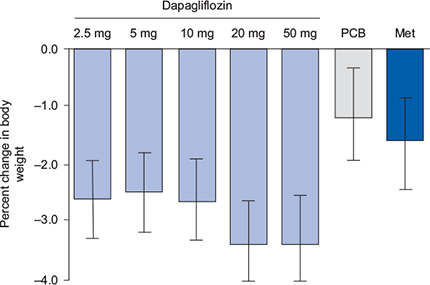
In a Phase 3 study, 546 patients not achieving adequate glycaemic control with metformin (at least 1500 mg/day) were randomized to adjunctive oral dapagliflozin 2.5 mg, 5 mg, 10 mg or placebo once daily for 24 weeks (Bailey et al., 2010). Mean HbA1c reductions from baseline were –0.67%, –0.70% and –0.84% (all P < 0.001) in the dapagliflozin 2.5 mg, 5 mg, and 10 mg groups, respectively, compared with -0.30% in the placebo group. Patients assigned to placebo lost a mean of 0.9 kg compared with 2.2 kg with dapagliflozin 2.5 mg, 3 kg with dapagliflozin 5 mg, and 2.9 kg with dapagliflozin 10 mg. Symptoms of hypoglycaemia occurred in similar proportions of patients in the dapagliflozin and placebo groups (approximately 3%). In addition to dapagliflozin, a number of other SGLT-2 inhibitors are in development, the most advanced of which is canagliflozin. However, at the time of writing no published papers were available on this agent. The clinical development of two agents, sergliflozin etabonate and remogliflozin etabonate, was halted at Phase 2 trials.
Safety and tolerability
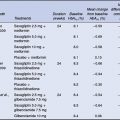
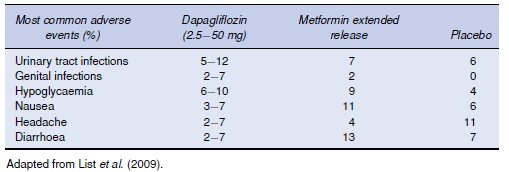
The side-effect profile of the SGLT-2 inhibitors is likely to depend on the ability to selectively inhibit SGLT-2 over SGLT-1, given that SGLT-1 inhibition is associated with glucose malabsorption and diarrhoea. To date, short-term safety data with the SGLT-2 inhibitors indicate that these agents appear well tolerated with no major difference in adverse events across treatment groups (Komoroski et al., 2009a; Komoroski et al., 2009b; List et al., 2009). The only exception is a higher incidence of genitourinary infections compared with placebo (Table 6.1) (Bailey et al., 2010; List et al., 2009). It is likely that the higher glucose concentrations in the urine may allow yeast organisms to flourish. The increase in urinary glucose excretion is not associated with a greater risk of hypoglycaemia or excessive losses of serum electrolytes and despite the increased urinary volume, few patients complain of excessive urination. The long-term safety of these agents is not yet known.
Table 6.1 Most common adverse events occurring with dapagliflozin therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree