The Expanding Role of Bisphosphonates and Novel Bone Agents in Breast Cancer
 ABSTRACT
ABSTRACT
Breast cancer is the most common malignancy among women in the United States. Many cancer treatments can have a detrimental effect on bone density, which is referred to as cancer therapy-induced bone loss (CTIBL). Chemotherapy use in premenopausal women often leads to premature menopause, resulting in accelerated loss of bone mineral density. Endocrine therapy, particularly aromatase inhibitors in postmenopausal women, can also decrease bone density. In addition, a majority of patients with metastatic breast cancer have bony involvement, leading to pain, increased risk of fracture, and other morbidities. A thorough understanding of factors associated with CTIBL and treatment options are essential to provide quality care to cancer survivors. This article summarizes risk factors, evaluation, and treatment options for CTIBL and bone metastases, with a focus on bisphosphonate therapy. Emerging data suggesting the bisphosphonates may be useful in both secondary and primary prevention of breast cancer are also discussed, and information regarding the current status of novel bone-targeted agents, including RANK ligand inhibitors, is presented.
Keywords: breast cancer, cancer therapy-induced bone loss, bisphosphonates, bone metastases
 INTRODUCTION
INTRODUCTION
The American Cancer Society estimates that there will be 207,090 new cases of invasive breast cancer diagnosed among women in the United States in 2010. Additionally, it is estimated that there are more than 2.5 million breast cancer survivors living in the United States. Standard adjuvant treatment for breast cancer may include chemotherapy, endocrine therapy, or a combination of the two. Both can have effects on circulating estrogen levels, which can lead to accelerated decreases in bone mineral density (BMD). In addition, it is clear that with improvements in the treatment of advanced disease, more women are living with metastatic disease. The data suggest that up to three fourths of women with metastatic breast cancer have bone involvement (1). Thus, bone health is an increasingly important issue in patients with early-stage and advanced breast cancer. This article will review the role of bisphosphonates and other bone-targeted agents in the management of both early-stage and metastatic breast cancer and will discuss the emerging role of bone-targeted agents in the prevention of disease recurrence.
 NORMOL BONE TURNOVER
NORMOL BONE TURNOVER
Bone is a dynamic tissue, undergoing continuous remodeling throughout life. Bone homeostasis is maintained by a balance of bone matrix and mineral resorption, mediated by osteoclasts, and bone formation, controlled by osteoblasts. The principal mechanism of action of bisphosphonates is inhibition of osteoclast-mediated bone resorption. Bone turnover is regulated by both locally acting cytokines, such as receptor activator of NF-κ B ligand (RANKL) and osteoprotegerin (OPG), and systemic hormones, including calcitonin, parathyroid hormone, insulin-like growth factor-1, and platelet-derived growth factor (2). A number of these pathways are the targets of agents that are in development for treatment of cancer. Systemically, parathyroid hormone, calcitonin, calcitriol and vitamin D work together to maintain serum calcium levels by regulating bone resorption. Steroid hormones such as glucocorticoids, estrogens, and androgens also contribute to bone growth and maintenance, and medications that affect hormone levels can result in changes in BMD. Local regulation of bone turnover is mediated via the OPG/ RANKL/RANK system. RANKL binds to RANK on the surface of osteoclastic precursor cells, causing maturation and differentiation, and is thus critical for survival of mature osteoclasts. OPG inhibits this process by binding RANKL, decreasing osteoclast formation and survival, resulting in decreased bone resorption. Cytokines such as tumor necrosis factor-α and interleukin-10 can also modulate bone turnover, primarily by stimulating production of macrophage colony-stimulating factor and increasing RANKL expression.
 EVALUATION OF BONE HEALTH
EVALUATION OF BONE HEALTH
Bone health is generally evaluated by BMD levels and is usually assessed using dual x-ray absorptiometry (DEXA) scanning of the hip and spine. Significant variation in DEXA measurements can exist owing to differences in calibration of individual DEXA scanners. Serial monitoring of BMD should be performed on the same piece of equipment using the same reference standards when possible. BMD can be expressed in absolute terms (grams per square centimeter) or, more commonly, is described on a relative scale as the difference in standard deviations from the expected BMD for the patient’s age and sex (z score) or from that of “young normal” adults of the same sex (T score). The World Health Organization (WHO) defines a normal BMD as that within one standard deviation of a young normal adult value (T score of ± 1.0); a T score −1.0 to −2.5 is considered osteopenia, and a T score of less than −2.5 constitutes osteoporosis (3). It is estimated that fracture risk doubles for each standard deviation reduction in BMD; however, many other factors, especially the patient’s age, also contribute to fracture risk (4). The WHO has developed a risk assessment tool called FRAX to estimate the risk of fracture to guide treatment decisions. This online tool incorporates BMD measures and a number of other clinical risk factors, including smoking, alcohol use, height, weight, and personal and family history, to provide estimates of 10-year risk of fracture (http://www.shef. ac.uk/FRAX/). Current Medicare guidelines recommend therapeutic intervention for patients with a 10-year FRAX risk of 3% for hip fractures and >20% for all major fractures. Many patients with cancer have an elevated risk for bone loss and fracture and should be evaluated periodically to assess the impact of their cancer treatment on bone mass. The most common oncologic and non-oncologic risk factors for osteoporosis are listed in Table 1.
Initial strategies for prevention and treatment of bone loss include lifestyle modification, such as performing regular weight-bearing, strength training, and balance exercises, avoiding tobacco, and limiting alcohol intake. In addition, all patients should be counseled to ensure adequate intake of calcium (at least 1,200 mg per day for all individuals older than 50 years of age and those with risk factors for osteoporosis) and vitamin D (800– 1,000 IU per day) (5). Therapeutic intervention (as described later) should be strongly considered for patients with a T score below −2.0, particularly in those with additional risk factors for fracture. The optimal duration of therapy for osteoporosis is unknown. Medications currently approved by the Food and Drug Administration (FDA) for treatment and prevention of osteoporosis are listed in Table 2.
TABLE 1
Risk factors for osteoporosis and osteoporotic fracture
| Non-Oncologic | Oncologic |
| Age | Treatment-induced menopause |
| Frequent falls | Hormonally active medication use |
| Family history of osteoporosis | Ovarian function suppression |
| Personal history of fragility fracture | Aromatase inhibitors |
| Rheumatoid arthritis | Anti-androgen therapy |
| Low body mass index | Other medications |
| Smoking | Glucocorticoids |
| Alcohol consumption | |
 EFFECTS OF BREAST CANCER THER APY ON BONE HEALTH
EFFECTS OF BREAST CANCER THER APY ON BONE HEALTH
The past decade has seen a number of advances in breast cancer therapy, leading to improved survival (6), and this has resulted in a growing number of women who are otherwise healthy, but who have received or are receiving cancer directed therapy. Cancer treatment-induced bone loss (CTIBL) is a recognized consequence of a number of treatments commonly used in early-stage breast cancer. In postmenopausal women, treatment with aromatase inhibitors (AIs) results in significant reduction in circulating estrogens, leading to accelerated decline in BMD. In premenopausal women, chemotherapy and LHRH agonist use can induce at least temporary cessation of ovarian function, with similar effects on bone density.
AI-Induced Bone Loss
AIs have been shown in multiple studies to decrease the risk of recurrence in postmenopausal patients with breast cancer when compared with tamoxifen, and have become increasingly common as adjuvant therapy for hormone receptor-positive postmenopausal breast cancer. AIs inhibit peripheral conversion of androgen to estrogen, resulting in a rapid decrease in circulating estrogen levels and thus accelerated bone loss and increased risk of fracture. Among AI-treated patients, older age, baseline osteopenia, and osteoporosis have been identified as risk factors for fracture. In the large adjuvant trials comparing AIs with tamoxifen, absolute rates of fracture among patients treated with AIs were 1% to 3% higher than those among tamoxifen-treated patients (7–9), corresponding to a relative increase in fracture risk of 35% to 55%. In the Anastrozole and Tamoxifen Alone or in Combination (ATAC) trial, in which patients were randomized to 5 years of anastrozole or tamoxifen, fracture rates after discontinuation of therapy were similar, suggesting that the increased risk of fracture with AI therapy may not be permanent (8). Unfortunately, because most of the adjuvant trials compare AI treatment with the standard of 5 years of tamoxifen, it is difficult to tease out the negative effects of the AIs on fracture risk from the known positive effects of tamoxifen. The MA.17 trial, which compares 5 years of letrozole with placebo after completion of 5 years of tamoxifen, shows a nonsignificant difference in the incidence of a new diagnosis of osteoporosis in the two arms (5.8% with letrozole compared with 4.5% with placebo, P = .07), and fracture rates were similar in the two groups (10). In a prospective substudy of the ATAC trial which assessed BMD changes among 197 randomized women, the median change in lumbar spine BMD was −6.08% in anastrozole-treated women and +2.77% in tamoxifen-treated women. Importantly, in that study, no patients with normal bone density at baseline developed osteoporosis during the 5 years of therapy, and only 5% of patients with osteopenia at baseline became osteoporotic (11). Taken together, these data imply that the difference in fracture rates between AIs and tamoxifen may be in part due to a bone-protective effect of tamoxifen rather than a detrimental effect of AIs.
TABLE 2
Agents currently FDA-approved for the treatment and prevention of osteoporosis
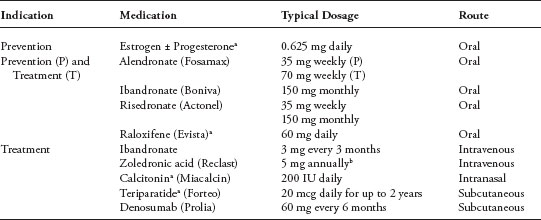
a Not routinely recommended for women with a history of breast cancer, particularly those with hormone receptor-positive disease.
b The dose of zoledronic acid in most studies evaluating its use in cancer treatment-related bone loss was 4 mg IV every 6 months; however, the dose that is FDA-approved for treatment of postmenopausal osteoporosis is 5 mg IV annually.
A number of recent studies have evaluated the impact of oral and intravenous bisphospho-nate therapy on BMD in women undergoing adjuvant AI therapy for breast cancer. In the Study of Anastrozole with the Bisphosphonate Risedronate (SABRE) trial, women receiving anastrozole were assigned to oral bisphosphonate therapy or placebo based on baseline BMD evaluation: those with normal bone density (T score > −1.0) received no additional therapy; those with mild osteopenia (T score between −1.0 and −2.0) were randomized to oral risedronate at a dose of 35 mg weekly versus placebo; and those with severe osteopenia or osteoporosis (T score of less than −2.0) were treated with risedronate. At 24 months, treatment with risedro-nate resulted in favorable effects on BMD (12). The Effect of Oral Ibandronate on Anastrozole-induced Bone Loss (ARIBON) trial used a similar design(13).Patients with a T score of −1.0 to −2.5 were randomized to either oral ibandronate or placebo, and those with a T score < −2.5 received ibandronate. The addition of ibandronate to anastrozole led to a significant increase in BMD at the spine and hip after 2 years of therapy, with the greatest change seen in women who had osteoporosis at study entry.
The Zometa-Femara Adjuvant Synergy trials (Z-FAST and Zo-FAST) evaluated the efficacy of upfront versus delayed initiation of zoledronic acid, a highly potent intravenous aminobisphosphonate, in preventing AI-associated bone loss (14). Patients were randomized to either upfront zoledronic acid at 4 mg intravenously every 6 months or delayed zoledronic acid (initiated at the same dosing only for patients who developed a lumbar spine or total hip T score of less than −2.0 or a nontraumatic fracture) in combination with letrozole 2.5 mg orally daily, calcium, and vitamin D. At 12 months, lumbar spine BMD was 5.2% higher in the upfront group than in the delayed group, though fracture rates were similar.
There are several important outstanding questions regarding treatment of CTIBL. No trials to date have compared oral with intravenous bisphosphonates for preservation of BMD in patients with breast cancer receiving AIs. However, the existing data suggest that both oral and intravenous bisphosphonates can mitigate the bone loss associated with AI therapy. Additionally, the primary endpoint in most studies was change in BMD, not fragility fracture, which may be a more clinically significant outcome. Lastly, the optimal duration of bisphosphonate therapy in combination with AIs has not been evaluated.
RANKL enhances osteoclast production and survival, resulting in increased bone resorption. Denosumab is a humanized monoclonal antibody to RANKL that has been studied in the setting of both postmenopausal osteoporosis and CTIBL. Its effect on AI-induced bone loss was assessed in a phase III placebo-controlled study in patients with breast cancer in the adjuvant setting (15). Patients on the treatment arm received 60 mg of denosumab subcutaneously every 6 months for 2 years. Study participants were stratified by duration of AI use. At 12 and 24 months, lumbar spine BMD increased by 5.5% and 7.6%, respectively, in the denosumab group as compared with the placebo group (P < .0001), and increases were also seen in BMD at the hip and radius. Changes in bone density were not significantly influenced by duration of AI therapy. Denosumab has also been studied for prevention and treatment of osteoporosis in non-cancer populations (16) and was recently FDA approved for the treatment of osteoporosis. Trials assessing the effect of denosumab on disease recurrence in the adjuvant setting are in the planning stages. Trials in the setting of metastatic bone recurrence are described later in this manuscript.
Chemotherapy-Induced Ovarian Failure
It is estimated that up 25% of women with breast cancer are premenopausal at the time of diagnosis. Premenopasual women with early-stage breast cancer who receive adjuvant chemotherapy generally experience at least temporary amenorrhea, and more than 50% will experience premature ovarian failure (17–19). The risk of ovarian failure increases with increasing age at diagnosis and also varies according to chemotherapy agents used and the dose and number of cycles of treatment (20). Several studies have reported accelerated bone loss as a consequence of chemotherapy-induced premature ovarian failure. In a prospective study by Shapiro et al., 71% of young women undergoing adjuvant chemotherapy experienced ovarian suppression, and in these patients a highly significant loss of bone density was seen in the lumbar spine at 6 months (21). Additional data suggest that bone loss associated with chemotherapy-induced menopause is several-fold higher than that seen with natural menopause or with AI treatment in postmenopausal women (5).
Oral clodronate and risendronate have both been studied in the setting of chemotherapy-induced ovarian failure. When compared with placebo, both agents resulted in about a 2% to 3% absolute improvement in BMD (22, 23). Zoledronic acid was evaluated in Cancer and LeukemiaGroup B (CALGB) 79809 study, in which premenopausal women beginning adjuvant chemo-therapy were randomized to immediate versus delayed administration of zoledronic acid. At 12 months, patients on the immediate treatment arm had a 2.6% improvement in BMD, whereas those on the delayed arm, who had not yet received zoledronic acid, experienced a 6.4% loss of BMD(24
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


