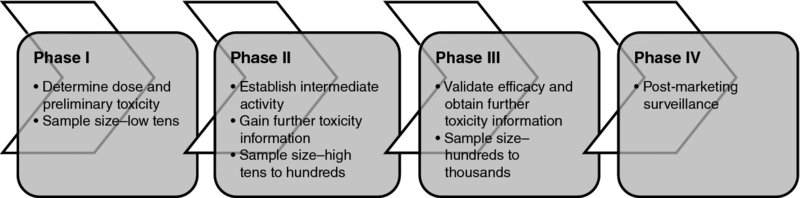
1 Sarah Brown, Julia Brown, Walter Gregory and Chris Twelves Traditionally, cancer drug development can be defined by four clinical testing phases (Figure 1.1): Figure 1.1 Four clinical phases of drug development. Presented in this way drug development may appear to be a straight line pathway, but this is often not the case in practice, with much more time and money invested in large phase III trials than in other stages of development. Likewise, the boundaries between the different stages of drug development are increasingly blurred. For example, many phase I trials treat an expanded cohort of patients at the recommended phase II dose often at least in part to demonstrate proof of principle or seek evidence of activity. In recent years a wide range of new ‘targeted’ cancer therapies have emerged with well-defined mechanisms of action directed at specific molecular pathways relevant to tumour growth and often anticipated to be used in combination with other standard treatments. This contrasts with cytotoxic chemotherapy from which the traditional four phases of cancer drug development emerged. Nevertheless, phase II cancer trials retain their pivotal position between initial clinical testing and costly, time-consuming definitive efficacy studies. The process from pre-clinical development to new drug approval typically takes up to 10 years and is estimated to cost hundreds of millions of dollars, although there is some uncertainty over the true costs (Collier 2009). Cytotoxic therapies, which lack a specific target and mechanism of action, often have a low therapeutic index, and historically have high rates of failure during drug development due to lack of efficacy and/or toxicity (Walker and Newell 2009). Although attrition rates for targeted cancer therapies appear lower than those of cytotoxic drugs, more drugs progress to expensive late stages of development before being abandoned in cancer than other therapeutic areas (DiMasi and Grabowski 2007). These worrying statistics have led to increased attention on clinical trial design, aiming to reduce the attrition rate and improve the efficiency of cancer drug development. This book focuses on the high-risk transition between phase II and III clinical trials and provides a practical guide for researchers designing phase II clinical trials in cancer. There is a clear need for phase II trials that more accurately identify potentially effective therapies that should move rapidly to phase III trials; perhaps even more pressing is the need for earlier rejection of ineffective therapies before they enter phase III testing. On this basis we aim to provide researchers with a detailed background of the key elements associated with designing phase II trials in patients with cancer, a thought process for identifying appropriate statistical designs and a library of available phase II trial designs. The book is not intended to be proscriptive or didactic, but instead aims to facilitate and encourage an interactive approach by the clinical researcher and the statistician, leading to a more informed approach to designing phase II oncology trials. Phase II trials in cancer are primarily designed to assess the short-term activity of new treatments and the potential to move these treatments forward for evaluation of longer-term efficacy in large phase III studies. In this respect, the term ‘activity’ is used to describe the ability of an investigational treatment to produce an impact on a short-term or intermediate clinical outcome measure. We distinguish this from the term ‘efficacy’ which we use to describe the ability of an investigational treatment to produce a significant impact on a longer-term clinical outcome measure such as overall survival in a definitive phase III trial. Cancer phase II trials are therefore invariably conducted in the metastatic or neo-adjuvant settings, where measurable short-term assessments of activity are more easily obtained than in the adjuvant setting. We focus on phase II trials in cancer, where assessments of ‘activity’ are usually not immediate and cure not achievable. Nevertheless, many of the statistical designs available for phase II cancer trials, and concepts discussed, may be applied to other disease areas. Phase II trials act as a screening tool to assess the potential efficacy of a new treatment. That broad description incorporates many different types of phase II trials including assessing not only traditional evidence of tumour response but also proof of concept of biological activity, selection between potential doses for further development, choosing between potential treatments for subsequent phase III testing and demonstration that the addition of a new agent to an established treatment appears to increase the activity of that treatment. In 1982 Fleming stated that ‘Commonly the central objective of phase II clinical trials is the assessment of the antitumor “therapeutic efficacy” of a specific treatment regimen’ (Fleming 1982). More recently the objective of a phase II trial in an idealised pathway has been described to ‘establish clinical activity and to roughly estimate clinical response rate in patients’ (Machin and Campbell 2005). Others have taken this a step further to claim ‘The objective of a phase II trial should not just be to demonstrate that a new therapy is active, but that it is sufficiently active to believe that it is likely to be successful in pivotal trials’ (Stone et al. 2007a). A common feature of phase II trials is that their aim is not primarily to provide definitive evidence of treatment efficacy, as in a phase III study; rather, phase II trials aim to show that a treatment has sufficient activity to warrant further investigation. The International Conference on Harmonisation (ICH) Guideline E8: General Considerations for Clinical Trials prefers to consider classification of study objectives rather than specific trial phases, since multiple phases of trials may incorporate similar objectives (ICH Expert Working Group 1997). The objectives associated with phase II trials in the ICH guidance are predominantly to explore the use of the treatment for its targeted indication; estimate or confirm dosage for subsequent studies; and provide a basis for confirmatory study design, endpoints and methodologies. Additionally, however, ICH notes that phase II studies, on some occasions, may incorporate human pharmacology (assessing tolerance; defining or describing pharmacokinetics/pharmacodynamics; exploring drug metabolism and interactions; assessing activity) or therapeutic confirmation (demonstrating/confirming efficacy; establishing a safety profile; providing an adequate basis for assessing benefit/risk relationship for licensing; establishing a dose/response relationship). These definitions have in common that oncology phase II trials act as an intermediate step between phase I testing on a limited number of patients to establish the safety of a new treatment and definitive phase III trials aiming to confirm the efficacy of a new treatment in a large number of patients. The specific aims of a phase II trial may, however, differ depending on the mechanism of action of the drug in question, the amount of information currently available on the drug and the setting in which it is being investigated (e.g. pharmaceutical industry vs. academia). Phase II trials can be broadly grouped into phase IIa and phase IIb trials. A phase IIa trial may be seen as seeking proof of concept in the sense of assessing activity of an investigational drug that has completed phase I development or may investigate multiple doses of a drug to determine the dose–response relationship. Phase IIa trials may be considered learning trials and be followed by a decision-making ‘go/no-go’ phase IIb trial to determine whether or not to proceed to phase III; phase IIb trials may include selection of a single treatment or dose from many and may include randomisation to a control arm.
Introduction
1.1 The role of phase II trials in cancer
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






