Rare Gastrointestinal Tumors
Van Karlyle Morris
Ben George
Anal
With approximately 9000 cases are expected to occur annually (1), squamous cell carcinoma of the anal canal is considered a rare malignancy, but nonetheless is one which continues to rise in incidence (2). Most cases are linked to prior infection with high-risk human papilloma virus (HPV)—most commonly, HPV-16 and HPV-18. Historically, systemic cytotoxic therapy has been utilized in the management of patients with advanced anal cancer. In recent years, molecular characterizations of this tumor type have elucidated possible targets for therapeutic intervention. This section details genomic and immune biomarkers of interest in the clinical management of patients with anal cancer.
Genomic Biomarkers
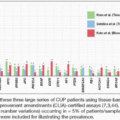
Whole-exome sequencing performed on a cohort of patients with metastatic anal cancer revealed activating mutations in the PIK3CA oncogene to be the most common alteration, present in 29% of cases (3). Amplifications in PIK3CA were seen in 88% of tumors. Commercially available gene sequencing panels that investigated across all stages of anal cancer have corroborated that PIK3CA is mutated in approximately 30% of all anal cancers (4,5). Interestingly, these findings are consistent with TCGA data that included other HPV-positive cancers including head/neck (6) and the cervix (7), both of which have PIK3CA mutations at similar frequencies. To date, no phosphoinositide 3-kinase (PI3K) inhibitors or mechanistic target of rapamycin (mTOR) inhibitors have been formally tested, specifically in patients with advanced anal cancer.
Unlike adenocarcinomas arising in the adjacent colon/rectum, anal cancers are characterized by rare mutations in the KRAS, NRAS, and BRAF oncogenes (3). Multiple series have reported that mutations in these genes occur in <5% of all anal cancers (4,5). Oncologists should recognize differences in biologic drivers and molecular profiling that distinguish colorectal cancer from nearby anal cancer when considering application of targeted therapies in the management of advanced cancers of the distal gastrointestinal tract. Given this predisposition for KRAS/NRAS/BRAF wildtype status for anal cancer, retrospective series have reported both safety/tolerability and antitumor activity of anti-epidermal growth factor receptor (EGFR) antibodies (mostly in combination with cytotoxic chemotherapy) (8), providing rationale for possible activity of this targeted therapy.
Immune Biomarkers
Anal cancers are also characterized by low tumor mutation burden (TMB), with multiple series estimating mean TMB below 10 mutations per megabase (Mb) of DNA sequenced (3,9). TMB-high status occurs in <10% of reported anal cancers (9). Although the U.S. Food and Drug Administration (FDA) has approved pembrolizumab in patients with advanced solid tumors featuring a TMB >10 mutations/Mb (10), prospective studies thus far have not validated TMB as a predictive biomarker for benefit with immunotherapy in patients with advanced anal cancer.
Anti-programmed cell death 1 (PD-1) antibodies have demonstrated modest efficacy in patients with metastatic anal cancer with responses in single-arm phase II clinical trials reading between 10% and 24% (11,12). Post hoc analyses have suggested increased benefit in patients whose tumors feature high expression of programmed death ligand 1 (PD-L1) and higher CD8+ T-cell infiltration in pretreatment tumor specimens (11). However, responses have also been observed in PD-L1-negative anal cancer patients by immunohistochemistry (12). At this time, PD-L1 has not been demonstrated to be a validated predictive biomarker for benefit with immunotherapy in patients with metastatic anal cancer.
| ||||||||||||
Small Bowel Cancers
Adenocarcinomas represent 25% to 40% of the 11,390 new cases of small bowel cancer reported annually in the United States (1,15,16). The incidence of adenocarcinoma is highest in the duodenum and decreases progressively throughout the rest of the small intestine (17,18). Systemic chemotherapy is the current mainstay of treatment for advanced small bowel adenocarcinomas (SBAs), but identification of predictive biomarkers to drive therapeutic decision-making is garnering increasing attention.
Mismatch repair deficiency (dMMR) status ranging from 1% to 16% has been reported for SBAs (19,20,21), although 7% to 10% of SBAs have high levels of TMB—defined as ≥20 mutations per megabase (21,22). A large series that prospectively analyzed samples from 317 patients with SBA identified TP53, KRAS, APC, SMAD4, PIK3CA, CDKN2A, and ARID1A as the most altered genes (>10% of cases), whereas ERBB2 alterations (mostly point mutations) and BRAF mutations (mostly non-V600E) were identified in 9.5% and 9.1% of cases, respectively (21). Another study identified a 22% incidence of DNA damage repair (DDR) gene alterations (ARID1A, ATM, ATR, BRCA1, BRCA2, CDK12, CHEK1, CHEK2, PALB2, and RAD51) in 666 patients with SBAs (22). Further, exome sequencing performed on 106 primary SBAs demonstrated four mutational signatures—signature 6, associated with mismatch repair (MMR) deficiency; signature 1A, associated with older age; and signatures 17 and U2, with no specific associations (23). The genomic profile of SBAs, even while harboring some similarities to those of colorectal cancer and gastric cancer, appears to be driven by disparate and distinct driver alterations meriting individualized treatment approaches (21,23).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree