Programmed Cell Death 1 (PD-1)/Programmed Cell Death Ligand 1 (PD-L1) Inhibitors
Jesse Hirner
Brittany A. Klein
Anna Kristine Dewan
Programmed cell death 1 (PD-1) is a receptor found on T lymphocytes that when activated, normally promotes immune self-tolerance. Tumors upregulate expression of its ligand, programmed cell death ligand 1 (PD-L1), to suppress CD8+ cytotoxic T-cell production, increase regulatory T-cell proliferation, and decrease production of inflammatory cytokines.1 PD-1 and PD-L1 inhibitors block tumor immune evasion mechanisms and are approved for use across diverse malignancies.1 PD-1 inhibitors include nivolumab, pembrolizumab, and cemiplimab. PD-L1 inhibitors include atezolizumab, avelumab, and durvalumab.
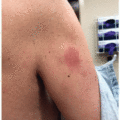
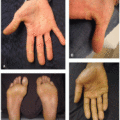
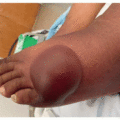
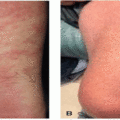
Given the immune dysregulation that occurs with use, a wide variety of cutaneous immune-related reactions to PD-1/PD-L1 inhibitors have been described. Eczematous eruptions (Figure 41.1) develop in up to 17% of patients and may be delayed up to 6 months from the start of therapy.2 Morbilliform eruptions (Figure 41.2) occur in up to 29% of patients, generally within 2 to 3 weeks of starting treatment, and tend to be self-limited.3 Psoriasiform eruptions (Figure 41.3) occur more quickly when a patient with preexisting psoriasis has a flare versus development of de novo skin disease and patients may have ocular, nail, or joint involvement (Figure 41.4).4 Lichenoid eruptions (Figure 41.5) may develop up to a year into therapy, and can involve the oral mucosa or skin folds5; bullous and hypertrophic variants may occur. Bullous reactions including bullous pemphigoid (BP)-like (most common) (Figure 41.6), mucous membrane pemphigoid-like, pemphigus-like and overlap presentations may occur. Onset is up to 20 months after treatment, and may be triggered by radiation.6,7 Urticarial or nonbullous pemphigoid should also be considered in the setting of severe eczematous dermatitis recalcitrant to therapies.8 Vitiligo-like depigmentation (Figure 41.7) may occur in up to 25% of melanoma patients and has a strong association with antitumor response to therapy in that setting6,9; it can also occur with other malignancies. Autoimmune connective tissue disease with dermatologic manifestations such as scleroderma, dermatomyositis, lupus erythematosus, and vasculitis may develop during treatment with PD-1/PD-L1 inhibition, or flare if preexisting6; the most common de novo cutaneous connective tissue disease enriched in this population appears to be subacute cutaneous lupus,10 and there are numerous reports of immunotherapy-induced eosinophilic fasciitis.11 Severe mucocutaneous adverse reactions including Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN)-like reactions (Figures 41.8 and 41.9), and drug reaction with eosinophilia and systemic symptoms (DRESS), while rare, have been described.9 Of note, unlike traditional SJS/TEN, which rapidly progresses, SJS/TEN-like reactions have been more often reported to begin atypically as morbilliform or lichenoid eruptions
that may evolve over weeks to months to full-thickness epidermal necrosis.12 The clinical trajectory of patients with cutaneous toxicities to PD-1/PD-L1 inhibitors should therefore be monitored carefully. Pruritus associated with PD-1/PD-L1 inhibition is common and may be severe and occur without primary cutaneous findings, although patients may develop nodular lesions from chronic scratching. Reactive keratoacanthomas or eruptive atypical squamous cell proliferations have also been described in patients treated with PD-1 inhibition (Figure 41.10).13,14 Typically occurring on the extremities, these lesions usually develop after a year of therapy.13,14
that may evolve over weeks to months to full-thickness epidermal necrosis.12 The clinical trajectory of patients with cutaneous toxicities to PD-1/PD-L1 inhibitors should therefore be monitored carefully. Pruritus associated with PD-1/PD-L1 inhibition is common and may be severe and occur without primary cutaneous findings, although patients may develop nodular lesions from chronic scratching. Reactive keratoacanthomas or eruptive atypical squamous cell proliferations have also been described in patients treated with PD-1 inhibition (Figure 41.10).13,14 Typically occurring on the extremities, these lesions usually develop after a year of therapy.13,14
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree