Cytotoxic T-Lymphocyte-Associated Protein 4 (CTLA-4) Inhibitors
Michael S. Chang
Rebecca I. Hartman
Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) functions as an immune checkpoint in negatively regulating T-cell function. T-cell activation requires costimulation via binding of B7 molecules with CD28 on the T-cell surface. CTLA-4 acts as a homolog with greater binding affinity for B7 than CD28, preventing downstream effects of T-cell activation including proliferation, survival, and differentiation.1 Inhibitors of CTLA-4, such as ipilimumab and tremelimumab, activate the immune system to target cancer cells and have been approved for use in metastatic melanoma with significantly improved survival outcomes.1
Despite these benefits, CTLA-4 inhibitors remain hindered by immune-related adverse events (irAEs); cutaneous irAEs are the most commonly experienced in nearly 45% of patients across pooled clinical trials.2 In addition, combination therapy with other immune checkpoint inhibitors (ICIs) results in more frequent eruptions, more severe and longer-lasting cutaneous irAEs.3,4 In metastatic melanoma patients, anti-CTLA-4 therapy demonstrated a dose-dependent relationship with higher-grade irAEs overall.5
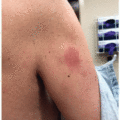
A pruritic morbilliform rash is one of the most frequent cutaneous irAEs observed with CTLA-4 inhibitors, with an estimated overall incidence of 24.3% for ipilimumab users (Figure 40.1). These rashes have been described as similar in appearance to morbilliform eruptions associated with common medications, such as antibiotics.2 Rashes associated with CTLA-4 inhibitors have also been morphologically described as reticular, erythematous, and edematous, affecting both the trunk and extremities.2
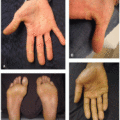
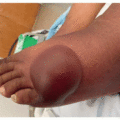
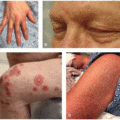
Other specific unique cutaneous irAEs reported with anti-CTLA-4 agents include psoriasis, Grover’s disease, eczematous eruptions (Figure 40.2), dermatomyositis, sarcoidosis, panniculitis (Figure 40.3), Sweet’s syndrome (Figure 40.4), lymphomatoid papulosis-like eruptions (Figure 40.5), and acneiform eruptions, although the relative incidences are not well established.3 These cutaneous eruptions may either be de novo or represent exacerbation of preexisting skin disease. In addition, relative to other ICIs, anti-CTLA-4 toxicities are less likely to affect the oral mucosa.3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree