MEK Inhibitors
Michael S. Chang
Rebecca I. Hartman
The mitogen-activated protein kinase (MAPK) pathway is highly implicated in aberrant signaling leading to carcinogenesis. Primary targets of this signaling cascade include RAS, RAF, MEK, and ERK with the latter two considered downstream of tyrosine kinase, RAS, and BRAF activation.1 As such, anticancer agents that inhibit MEK1 and MEK2 proteins strategically offer an alternative therapeutic strategy for malignancies associated with MAPK pathway dysregulation. Common examples of MEK inhibitors include selumetinib, trametinib, binimetinib, and cobimetinib.
Compared to BRAF inhibitors, which inhibit the MAPK pathway upstream of MEK proteins, MEK inhibitors possess a unique side effect profile that more closely resembles that of epidermal growth factor receptor (EGFR) inhibitors. In addition, when used in combination with BRAF inhibitors, MEK inhibitors have been shown to not only improve treatment efficacy but also exhibit reduced MEKi and BRAFi toxicity.2,3
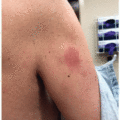
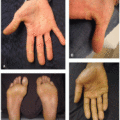
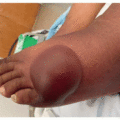
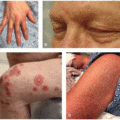
The most common cutaneous adverse events associated with MEK inhibitors are exanthematous morbilliform eruptions, acneiform eruptions (Figure 30.1), photosensitivity (Figure 30.1C), and xerosis with pruritus.4 Specifically with regards to acneiform eruption, the clinical course is highly similar to that of EGFR inhibitors, primarily affecting the head and neck, as well as upper body. Eruptions may also be complicated by pruritus and superinfection, most commonly with Staphylococcus aureus.4,5 Other cutaneous toxicities caused by MEK inhibitor therapy include hair and nail disorders, presenting as mild alopecia, hair depigmentation, paronychia, fissures (Figure 30.2), and trichomegaly.4,5,6,7 A rare and unique eruption secondary to MEKi has been termed “MEKi-induced dusky erythema” and patients present with violaceous and somewhat targetoid macules, patches, and plaques8 (Figure 30.3). It has been reported with combination therapy, all patients respond to topical or oral steroids, and patients may be rechallenged with clinical follow-up. Importantly, systemic side effects of MEKi include edema (Figure 30.4) and fever, and when presenting together or coupled with an eruption can be mistaken for infection or drug reaction with eosinophilia and systemic symptoms syndrome.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree