Toxicities from anticancer therapies affecting the hands and feet are common and can be divided into three main patterns with distinct clinical presentations and inciting chemotherapeutic agents. An overview of clinical features, culprit chemotherapeutic agents, and management strategies for cutaneous toxicities of the hands and feet is summarized in
Table 15.1.
Hand-foot syndrome (HFS), also referred to as palmoplantar erythrodysesthesia or acral erythema of the hands and feet, is considered a variant of toxic erythema of chemotherapy (TEC). It is most often encountered in the setting of treatment with cytotoxic chemotherapy, most commonly with antimetabolites and antitumor antibiotics; reported incidence varies depending on the causative agent. The highest incidence of HFS has been observed with capecitabine and pegylated liposomal doxorubicin at 50% to 60% and 30% to 50%, respectively.
1,
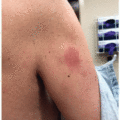
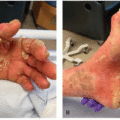
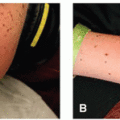
2 HFS is characterized by a prodrome of dysesthesia typically described as tingling or burning pain, followed by the appearance of symmetric, well-demarcated erythematous and edematous plaques localized to a palmoplantar distribution (
Figure 15.1). HFS usually occurs within the first two cycles of therapy (between 2 and 21 days), although it has been reported up to 10 months later after treatment onset.
1,
3 The palms, including lateral aspect of fingers and distal fat pads, are more commonly affected than the soles; as this is an acral presentation of toxic erythema of chemotherapy, infrequently, other sites, such as intertriginous areas, can also be affected.
1
The spectrum of clinical severity may vary from dysesthesia and minimal skin changes, to painful vesicles and bullae, with eventual desquamation, erosion, and ulceration.
3 Preventative strategies include cooling techniques (such as placement of ice packs over extremities before, during, and after infusions) or favoring administration of chemotherapeutic agents as bolus rather than prolonged infusion or continuous oral agents. Efficacy of cooling varies, with good responses in the setting of doxorubicin. Further management efforts, aimed at the degree of clinical severity, may include high-potency (class I) topical corticosteroid ointment for minimal skin changes (eg, erythema, edema, hyperkeratosis) with additional oral analgesic agents as needed if associated with pain and limitation on activities of daily life. Patients with more severe skin changes such as blistering or bleeding may be offered prophylaxis with dexamethasone (eg, 8 mg twice daily × 5 days starting on the day before infusion, then 4 mg twice daily × 1 day
followed by 4 mg once daily × 1 day). In the setting of HFS secondary to capecitabine, celecoxib has been shown to be an effective treatment. Indications to hold therapy include severe skin changes with blistering, bleeding, edema with pain, and limiting self-care activities of daily living. Of note, in severe cases of HFS, secondary infection is possible. Overall, HFS is a self-limiting, non-life-threatening condition with improvement of symptoms expected 1 to 2 weeks after stopping treatment. Permanent sequelae are rare but may include loss of fingerprints as a result of epidermal destruction, acral hyperpigmentation or necrosis, palmoplantar keratoderma, or scleroderma-like changes particularly in the setting of chronic HFS induced by capecitabine.