Highlights
- •
This study is the first to explore the efficacy and safety of the docetaxel, cisplatin, and capecitabine (TPC) regimen compared to GP and TPF.
- •
Our findings indicate that the TPC regimen has comparable therapeutic effects to GP and TPF while demonstrating good safety, suggesting its potential as a new IC regimen for LA-NPC.
- •
We further conducted subgroup analyses on patients with different T classifications and clinical stages, confirming that the efficacy of TPC was comparable to that of GP and TPF across all subgroups.
Abstract
Background and objectives
Gemcitabine plus cisplatin (GP) and docetaxel plus cisplatin plus fluorouracil (TPF) are induction chemotherapy (IC) regimens for locally advanced nasopharyngeal carcinoma (LA-NPC). The oral convenience of capecitabine presents its potential as a fluorouracil substitute in the TPF regimen, which has yet to be thoroughly investigated. This study aims to compare the efficacy and safety of the docetaxel, cisplatin, and capecitabine (TPC) with GP and TPF in LA-NPC.
Methods
A retrospective analysis was conducted on newly diagnosed stage III-IVa nasopharyngeal carcinoma patients who received GP, TPC, or TPF induction chemotherapy followed by concurrent chemoradiotherapy (CCRT) between February 2019 and December 2021. A comparison of the prognostic outcomes and associated adverse reactions among patients receiving different IC regimens. Multivariate Cox regression was applied to analyze independent prognostic factors, and subgroup survival analyses were conducted based on these factors.
Results
A total of 291 LA-NPC patients were included, with 70 receiving TPC, 119 receiving GP, and 102 receiving TPF. Kaplan-Meier survival analysis indicated no significant differences in OS, PFS, LRFS, and DMFS among the 3 groups. Multivariate Cox regression identified T classification and clinical stage as independent prognostic factors. Subgroup analyses revealed no significant differences in OS and PFS between the 3 groups across T1-2 and T3-4 classifications or III and IVa stages.The TPC group exhibited lower incidence rates of treatment-related acute toxicity reactions, including grade 3–4 toxicities.
Conclusion
The TPC induction chemotherapy regimen demonstrates comparable efficacy to GP and TPF, while maintaining a favorable safety profile.
Introduction
Nasopharyngeal carcinoma (NPC) is a head and neck malignancy with distinct geographical characteristics, exhibiting the highest incidence rates in Southeast Asia and southern China . Due to the subtlety of early symptoms, over 70 % of patients present with locally advanced disease at the time of diagnosis . Intensity-modulated radiotherapy (IMRT) is fundamental in the treatment of NPC, significantly improving the prognosis of patients compared to those receiving 2D radiotherapy .
In the comprehensive treatment of NPC, interventional therapy (IC) plays a crucial role. It not only eliminates tumor micro-metastases that may go undetected through imaging but also reduces the risk of poor prognosis. Additionally, IC alleviates tumor burden prior to radiotherapy, enhancing both radiosensitivity and tolerance . IC combined with concurrent chemoradiotherapy (CCRT) is currently the most common treatment approach for locally advanced NPC (LA-NPC). However, the optimal IC regimen remains a topic of debate. Compared to other regimens, the combinations of gemcitabine plus cisplatin (GP) and docetaxel plus cisplatin plus fluorouracil (TPF) have been validated by multicenter Phase III clinical trials, retrospective studies, and meta-analyses, demonstrating superior efficacy in improving outcomes for LA-NPC . These regimens are now widely adopted in clinical practice.
In the TPF regimen, 5-fluorouracil (5-FU) is administered intravenously as a chemotherapeutic agent. It exerts its effects by incorporating into DNA and RNA, thereby inhibiting their metabolism and effectively blocking the proliferation of cancer cells and protein synthesis . For nearly the past 50 years, it has been a cornerstone chemotherapeutic agent in the treatment of colorectal cancer . However, the prolonged intravenous infusion of 5-FU in the TPF regimen may lead to venous thromboembolism (VTE) . A study indicated that while the TPF regimen demonstrates better efficacy compared to the docetaxel and cisplatin (TP) and cisplatin and fluorouracil (PF) combinations, it is associated with a higher incidence of adverse effects . In contrast, capecitabine, as a prodrug of 5-FU, is administered orally and converted to 5-FU in the body, offering lower toxicity and greater convenience of use . Current research has demonstrated that capecitabine, in combination with taxanes and cisplatin, can serve as a first-line chemotherapy regimen for recurrent and metastatic nasopharyngeal carcinoma, as well as stages IVa and IVb . Docetaxel is a second-generation taxane antitumor agent that demonstrates enhanced antitumor activity and lacks cross-resistance . Currently, there is a lack of research investigating whether capecitabine can replace 5-FU in combination with docetaxel and cisplatin for the treatment of LA-NPC. This study aims to analyze the differences in prognostic value and toxicity responses during induction chemotherapy between the TPC regimen and the GP and TPF regimens, ultimately exploring the optimal induction chemotherapy strategy for LA-NPC.
Materials and methods
Study population
This study included patients diagnosed with LA-NPC at the Guangxi Medical University Cancer Hospital from February 2019 to December 2021. Inclusion criteria were as follows: 1. Pathologically confirmed NPC; 2. Restaged according to the 8th edition of the American Joint Committee on Cancer (AJCC)staging criteria 8th edition as stage III-IVa; 3. Patients received IC plus CCRT; the IC regimens included GP, TPC, or TPF, administered every three weeks; the concurrent chemotherapy (CC) regimen consisted of single-agent cisplatin, also given every three weeks; 4. Patients in good general condition with a KPS score > 70; 5. Comprehensive clinical and baseline data available. Exclusion criteria included: 1. Pregnancy or serious diseases that may affect the patient’s prognosis; 2. Recent use of medications that might impact immune status; 3. Dual cancers; 4. Prior radiotherapy or chemotherapy for head and neck cancer; 5. Receipt of adjuvant chemotherapy, maintenance therapy, or immunotherapy following IC + CCRT; 6. Lack of properly recorded toxicities during treatment. This study is a retrospective analysis, with all patient data anonymized, exempting the need for informed consent.
Treatment
All patients received treatment according to the IC + CCRT regimen. The IC regimens included: GP (Gemcitabine 1000 mg/m 2 on days 1 and 8 + Cisplatin 80 mg/m 2 on day 1); TPC (Docetaxel 60 mg/m 2 on day 1 + Cisplatin 60 mg/m 2 on day 1 + Capecitabine 850 mg/m 2 bid from days 1 to 14); and TPF (Docetaxel 60 mg/m 2 on day 1; Cisplatin 60 mg/m 2 on day 1; and continuous intravenous infusion of 5-Fu 600 mg/m 2 over 120 h). This was administered for a total of 2 to 3 cycles. CC regimen: Cisplatin (100 mg/m 2 ) was administered on day 1, days 1–2, or days 1–3, depending on the patient’s specific condition, for a total of 1–3 cycles. The delineation of radiation therapy target volumes and organs at risk was performed in accordance with the International Commission on Radiation Units and Measurements (ICRU) Reports 50 and 62 standards, and reviewed by at least two radiation oncologist of deputy chief level or higher. The prescribed doses for target volumes were as follows: GTVnx 69.96–72.60 Gy (33 fractions), GTVnd or CTVnd 62.70–72.60 Gy (33 fractions), CTV1 60.00–64.00 Gy (33 fractions), and CTV2 54.00–57.60 Gy (33 fractions).
Endpoints and follow-up
The primary study endpoint is overall survival rate (OS), defined as the time from treatment initiation until death from any cause. Another primary endpoint is progression-free survival (PFS), indicating the time from treatment start to disease progression or patient death. Secondary endpoints include relapse-free survival (RFS), which measures the interval from treatment initiation to disease recurrence, and distant metastasis-free survival (DMFS), defined as the time from treatment start to the occurrence of distant metastasis. Toxicity assessments utilize the World Health Organization Adverse Drug Reaction (WHO-ADR) system. Follow-up will be conducted through phone calls, outpatient visits, and inpatient reviews. Patients will be followed up every 3 months for the first 2 years, every 6 months from years 3 to 5, and annually thereafter. Recurrence and post-treatment metastasis will be diagnosed through symptoms, physical examinations, nasopharyngoscopy, pathology, and imaging studies such as Computed Tomography (CT) and Magnetic Resonance Imaging (MRI).
NPC recurrence is defined as the presence of a pathologically confirmed NPC with a complete response post-radiotherapy, occurring in the same region as the original tumor six months after treatment. Post-treatment metastasis diagnosis relies on detailed medical history, symptoms, physical findings, characteristic imaging results, and EBV DNA testing in high-risk patients, with definitive diagnosis confirmed by pathology from the sites of recurrence.
Statistical analysis
Statistical analysis in this study was conducted using SPSS 25 for Windows (SPSS, Chicago, IL). Age was represented by the median as the cut-off value. Baseline characteristics and adverse reactions associated with different IC regimens were compared using χ2 or Fisher’s exact test. Kaplan-Meier survival curves were generated using R software (version 4.0.3) to assess patient prognosis, with the log-rank test employed for rate comparisons. Multivariate Cox regression was utilized to identify independent risk factors for patients. To assess the presence of multicollinearity in multivariate regression analysis, the variance inflation factor (VIF) is calculated.Subgroup survival analyses were performed to evaluate the benefits of different IC regimens based on identified independent risk factors. A p -value of < 0.05 was considered statistically significant.
Results
Patient characteristics
A total of 291 patients with LA-NPC from 2019 to 2021 were retrospectively included in this study. Among the total cohort, there were 217 males (74.6 %) and 74 females (25.4 %). The median age at follow-up was 46 years(ranging from 12-72 years). The median follow-up duration was 45.6 months (ranging from 5 to 65 months). The patients received different IC regimens: 119 (40.9 %) received GP, 70 (24.1 %) received TPC, and 102 (35.0 %) received TPF. Detailed baseline characteristics of the patients are presented in Table 1 . Except for clinical staging, which showed differences among the various IC groups, no other baseline characteristics demonstrated statistically significant differences ( p > 0.05).
| Characteristic | Total cohort(n = 291) | GP(n = 119) | TPC(n = 70) | TPF(n = 102) | p -value | |
|---|---|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | No. (%) | |||
| Age(years) | ≤46 | 152 (52.2) | 68 (57.1) | 32 (45.7) | 52 (51.0) | 0.300 |
| >46 | 139 (47.8) | 51 (42.9) | 38 (54.3) | 50 (49.0) | ||
| Gender | male | 217 (74.6) | 89 (74.8) | 52 (74.3) | 76(74.5) | 0.997 |
| female | 74 (25.4) | 30 (25.2) | 18 (25.7) | 26 (25.5) | ||
| Family history | No | 240 (82.5) | 95 (79.8) | 54 (77.1) | 91 (89.2) | 0.076 |
| Yes | 51 (17.5) | 24 (20.2) | 16 (22.9) | 11 (10.8) | ||
| Pathology | K-NPC/Basaloid SCC | 9 (3.1) | 3 (2.5) | 4 (5.7) | 2 (2.0) | 0.385 |
| NK-NPC | 282 (96.9) | 116 (97.5) | 66 (94.3) | 100 (98) | ||
| T classification | T1-2 | 89 (30.6) | 39 (32.8) | 23 (32.9) | 27 (26.5) | 0.535 |
| T3-4 | 202 (69.4) | 80 (67.2) | 47 (67.1) | 75 (73.5) | ||
| N classification | N0-1 | 75 (25.8) | 32 (26.9) | 16 (22.9) | 27 (26.5) | 0.813 |
| N1-2 | 216 (74.2) | 87 (73.1) | 54 (77.1) | 75 (73.5) | ||
| Clinical stage | C3 | 111 (38.1) | 37 (31.1) | 41 (58.6) | 33 (32.4) | <0.001 |
| C4 | 180 (61.9) | 82 (68.9) | 29 (41.4) | 69 (67.6) | ||
| Circles of IC | 2 | 13 (4.5) | 4 (3.4) | 2 (2.9) | 7 (6.9) | 0.343 |
| 3 | 278 (95.5) | 115 (96.6) | 68 (97.1) | 95 (93.1) | ||
| CCD (mg/m 2 ) | <200 | 17 (5.8) | 4 (3.4) | 7 (10) | 6 (5.9) | 0.171 |
| ≥200 | 274 (94.2) | 115 (96.6) | 63 (90) | 96 (94.1) |
Survival analysis and multivariate regression analysis
The 3-year survival outcomes for patients in the GP, TPC, and TPF groups were calculated. As shown in Figure 1 , the 3-year OS rates for the GP, TPC, and TPF groups were 88.6 %, 86.0 %, and 84.0 %, respectively. The PFS rates were 89.7 %, 87.0 %, and 84.0 %, while the RFS rates were 97.1 %, 98.6 %, and 96.8 %. The DMFS rates were 93.0 %, 88.3 %, and 92.7 %. The log-rank test indicated no statistically significant differences in OS, PFS, RFS, or DMFS among patients receiving different IC regimens during the follow-up period ( p > 0.05).
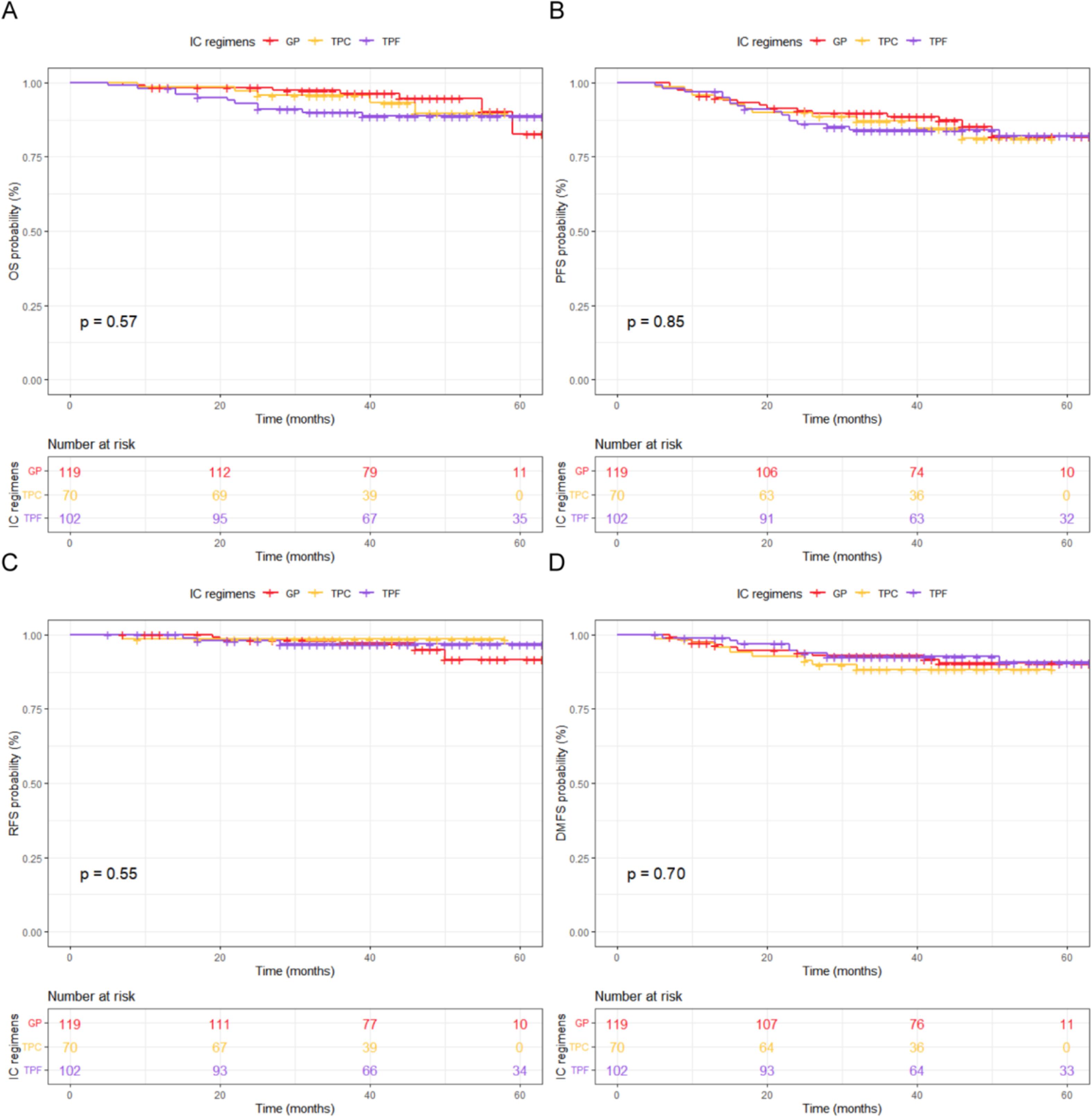
Based on the primary endpoints of OS and PFS, multivariate Cox regression analysis revealed that clinical staging was an independent prognostic factor affecting OS, while T classification and clinical stage were identified as independent prognostic factors for PFS. N classification, IC regimen, and the cumulative cisplatin dose (CCD) of CC did not serve as independent prognostic factors for LA-NPC patients in this study ( Table 2 ). A collinearity analysis was performed on all factors included in the multivariate Cox regression analysis for OS and PFS. The results showed that the VIF values of all prognostic factors were close to 1, indicating no collinearity issues.