Highlights
- •
Of PC patients on continuous ADT in Spain, 4.8% had CRPC with unknown metastatic status (MX).
- •
Most CRPC-MX patients had not been treated with curative intent at initial diagnosis despite ECOG ≤1.
- •
Most patients with CRPC-MX are not followed-up based on guidelines’ recommendations.
- •
PSA measurements, visits, and imaging scans increased after CRPC-MX identification.
- •
Identification of CRPC-MX patients improved physicians’ adherence to guidelines.
Abstract
Background
Early identification and management of metastases in prostate cancer (PC) patients is crucial. This study aimed to describe the nonpharmacological management and characteristics of patients with castration-resistant PC with unknown metastatic status (CRPC-MX) and estimate their prevalence in Spain.
Methods
Cross-sectional, multicenter, real-world study including adult (≥18 years) CRPC-MX patients from 46 Urology services. In a first phase, patients on continuous ADT for ≥6 months were screened and classified as hormone-sensitive PC (HSPC), castration-resistant PC (CRPC), and unknown hormonal status, with metastases (M1), without (M0) and unknown metastatic status (MX) using an ad hoc designed algorithm. In Phase 2, 15 months (m) after Phase 1, all patients on ADT were reviewed and reclassified again using the algorithm.
Results
Among 6169 eligible PC patients, 294 (4.8%) were classified as CRPC-MX, which decreased to 179 of 4050 (4.4%) 15 m after study initiation. We included 103 CRPC-MX patients with a median age at diagnosis of 75.4 years (IQR: 67.8, 80.4); 26 (25.2%) lacked a histological diagnosis, and only 25 (24.5%) received treatment with curative intent, despite ECOG being ≤1 at inclusion in 83.5%. In the 15 m before inclusion, most CRPC-MX patients had <5 prostate-specific antigen (PSA) determinations (80.6%) and no imaging (63.1%). After CRPC-MX identification (15 m after inclusion), metastatic status was assessed in 55.4%, with an increased number of patients with ≥5 PSA determinations ( P = 0.0357), visits per patient (P < 0.0001), patients with some imaging test ( P < 0.0001), imaging tests/patient ( P < 0.0001), and visits to onco-urology specialized consultation units (52.0% before and 79.2% after).
Conclusion
A substantial proportion of PC patients on ADT in the real-world setting are not appropriately followed up. Identification of CRPC-MX patients raised awareness among physicians and improved their adherence to guidelines, resulting in improved care for these patients.
1
Introduction
Prostate cancer (PC) is a heterogeneous disease generally associated with elevated prostate-specific antigen (PSA) levels [ ]. PC is one of the most common cancers in men worldwide, second to lung cancer [ ], and was the fifth leading cause of cancer death among men in 2020 [ ]. In Spain, of the estimated new cancer cases in men in 2023, 29,002 (18.29%) were estimated to be PC [ ].
Most prostate tumors are detected at an early and localized stage, when various curative treatment strategies are possible [ ], whereas patients with metastatic advanced disease will receive continuous androgen deprivation therapy (ADT) [ ]. Most patients receiving ADT show PSA response and are classified as hormone-sensitive PC (HSPC). However, the disease will progress to a state known as castration-resistant PC (CRPC), with increased PSA despite serum testosterone levels in the castration range [ ]. CRPC represents a heterogeneous clinical entity, from patients with an asymptomatic elevation of PSA and good functional status to patients with significant debilitating symptoms and rapidly progressive disease [ ]. PC patients treated with ADT can be further classified based on metastatic status into metastatic (M1) and nonmetastatic (M0).
For CRPC patients, guidelines recommend a PSA determination every 3 months and imaging tests at CRPC diagnosis and then at each doubling of PSA levels [ ]. However, if the recommended imaging tests are not properly performed, metastatic status cannot be assessed (CRCP-MX) [ ]. In Spain, the prevalence of CRPC patients with unknown metastatic status (CRPC-MX) was estimated in small patient series [ , ]. However, data regarding the real-world CRPC-MX prevalence across the Spanish territory are lacking. Moreover, the gaps in the routine follow-up of PC patients leading to an MX status and the factors potentially associated, such as patients’ clinical characteristics, are unknown.
Identifying the metastatic status is crucial since metastases are associated with tumor-related complications, which could be avoided or delayed with the appropriate treatment approach and follow-up [ ]. Therefore, investigating the current prevalence of CRPC-MX and the management leading to this status is essential for early identification and management of metastatic patients. This cross-sectional, multicenter, and real-world study aimed to describe the nonpharmacological management and characteristics of CRPC-MX patients, and to estimate their prevalence in Spain.
2
Material and methods
2.1
Study design and population
This was a cross-sectional, multicenter, noninterventional study including patients with CRPC-MX followed up in Urology services of 46 participating centers. Eligible patients were adults (≥ 18 years) with clinical or histological PC diagnosis undergoing continuous ADT for at least 6 months before inclusion. Exclusion criteria are summarized in the supplementary methods. Data were collected from clinical records, and all patients provided their informed consent before study inclusion.
The study was conducted in 2 phases. In Phase 1 (September 2020–December 2020), eligible patients were screened and classified based on their hormone status as HSPC, CRPC, and unknown hormonal status ( i.e. , invalid testosterone, defined as levels >50 ng/dL or not determined) and based on their metastatic status as metastatic (M1), nonmetastatic (M0), and unknown metastatic status (MX) using an algorithm designed ad hoc based on clinical guidelines [ ]. CRPC was defined as castration levels of serum testosterone (<50 ng/dL or 1.7 nmol/l) and 3 consecutive PSA increases in intervals of at least one week, of which 2 had to be ≥ 50% over nadir and > 2 ng/ml. Unknown metastasis status was defined based on RADAR guidelines recommendations [ ] as summarized in the supplementary methods. Identified CRPC-MX patients who signed the informed consent were included and baseline and follow-up data corresponding to the 15 months before their inclusion were collected. In Phase 2 (December 2021–May 2022), 15 months after Phase 1, all patients on ADT were reviewed and classified again using the algorithm. Data from the previously identified CRPC-MX group (“study patients”) regarding their nonpharmacological management were also collected. Fig. S1 depicts a diagram of the study design.
This study was conducted according to the Helsinki Declaration (Fortaleza, 2013) and following the Personal Data Protection local Law (Law 3/2018 December 5th) and the European General Data Protection regulations (EC 2016/679). The Research Ethics Committees of the participating sites approved the study protocol.
2.2
Objectives and variables
The primary objective of this study was to describe the nonpharmacological management of CRPC-MX patients during the 15 months before study inclusion and the real-world prevalence of the CRPC-MX condition in Spain. The secondary objectives were to describe the clinical and demographic characteristics of CRPC-MX patients from PC diagnosis and the nonpharmacological management and the change in CRPC-MX prevalence 15 months after study inclusion.
For patient classification according to hormonal and metastatic status in Phases 1 and 2, we considered PC diagnosis, testosterone and serial PSA determinations (hormonal status), and imaging data (imaging type, date, and outcome [metastatic status]). For CRPC-MX patients, we collected data regarding demographics, clinical characteristics, as well as initial disease diagnosis, management, and systemic treatments. Data related to nonpharmacological management were collected during the 15 months before and after study inclusion in Phases 1 and 2 ( Fig. S1 ), including PSA (values and frequency of determinations), number of follow-up visits, type of treating physician (general urologist or onco-urologist), and imaging data. In Phase 2 (15 months after study inclusion), we also considered the metastatic status and PC treatment.
2.3
Statistical analysis
Owing to the limited data on CRPC-MX prevalence, the sample size of this study could not be anticipated. The sample size was estimated based on a previous study estimating a prevalence of CRPC-MX of 0.0048% in Castellón, Spain [ ], among the general population. We calculated that 10,582,986 individuals were needed to estimate the prevalence of CRPC-MX in Spain with a 7.5% relative maximum error and a 95% confidence level, hypothesizing a total study population of 600 individuals with CRPC-MX.
Categorical data were described using frequencies and percentages, and continuous data were described using the mean and standard deviation (SD) and the median and interquartile range (IQR: Q1, Q3). Data from the 15 months before and after study inclusion were compared using the Wilcoxon test for paired continuous variables and the McNemar test for categorical variables. Statistical significance was set at a 2-sided α < 0.05. All statistical analyses were performed using the statistical package SAS® version 9.4.
3
Results
3.1
Prevalence of CRPC-MX
At study initiation (Phase 1), 6169 patients undergoing treatment with continuous ADT for ≥6 months were classified according to their hormone and metastatic status ( Fig. 1 ). A total of 1761 (28.6%) patients were CRPC, 3648 (59.1%) HSPC, and 760 (12.3%) had unknown/invalid hormonal status. Globally, the metastatic status was unknown in 2902 (47.1%) patients. Both hormonal and metastatic status were unknown in 559 (9.1%) patients.
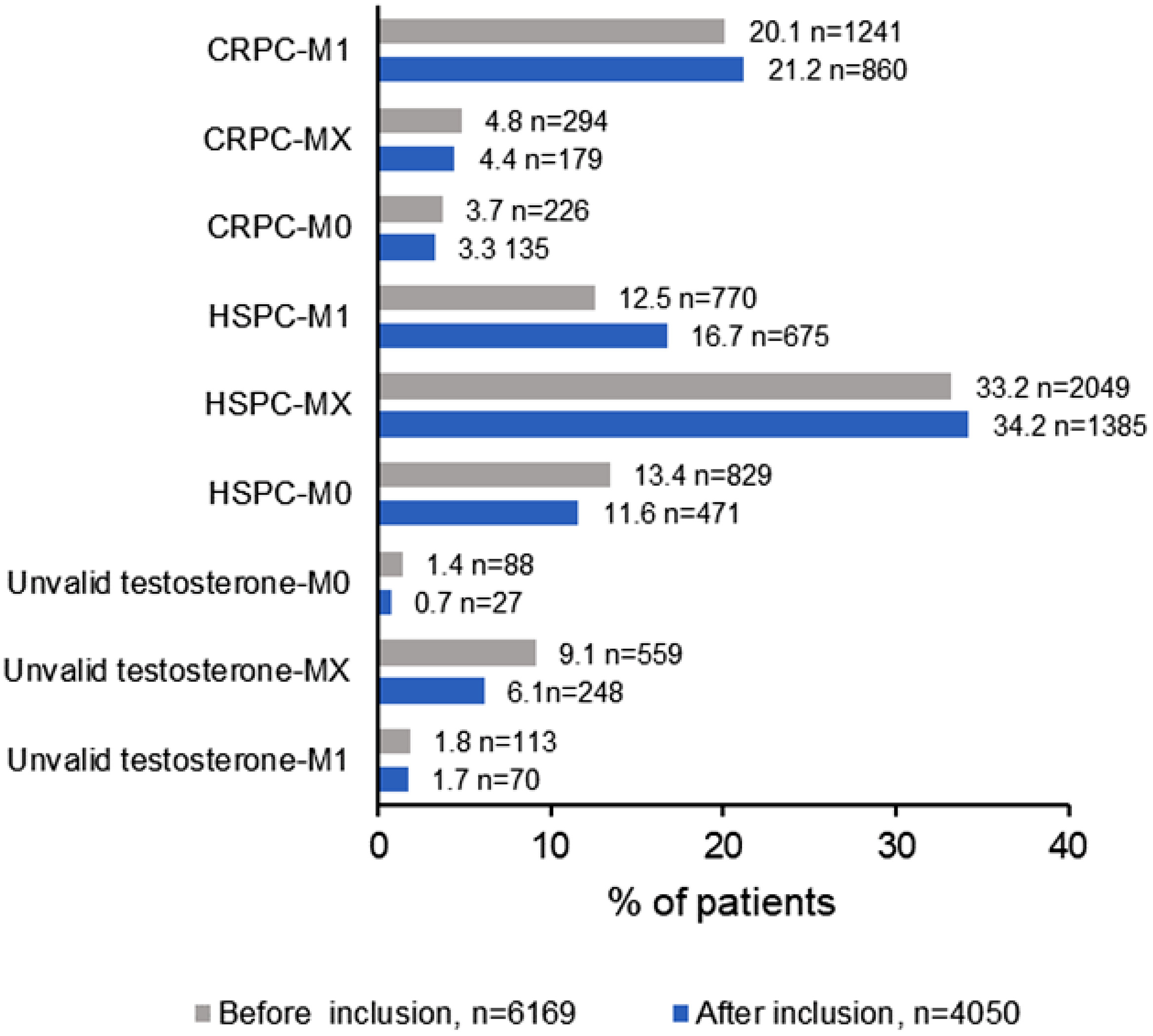
CRPC patients were classified as M1 (n = 1241, 20.1%), M0 (n = 226, 3.7%) and MX (n = 249, 4.8%). Patients with HSPC with unknown metastatic status were the most prevalent (n = 2049, 33.2%).
During Phase 2 (15 months after phase 1), 4050 patients on continuous ADT were classified again according to their hormone and metastatic status. The prevalence of CRPC-MX decreased modestly from 4.8% (n = 294) to 4.4% (n = 179) ( Fig. 1 ). Conversely, the prevalence of HSPC-MX and HSPC-M1 increased, with a concomitant decrease in the prevalence of HSPC-M0. In contrast, the prevalence of patients with unknown hormonal status (invalid testosterone or not determined) decreased for all metastatic status, although to a varying degree ( Fig. 1 ).
3.2
Characteristics of CRPC-MX patients at initial diagnosis and inclusion
Of the 327 patients initially classified as CRPC-MX, 142 provided informed consent and 39 were excluded for not meeting the inclusion criteria, resulting in a study population of 103 patients valid for analysis ( Fig. S2 ). Table 1 summarizes the characteristics of the study population at initial diagnosis and study inclusion. Median age at diagnosis was 75.4 years (IQR: 67.8, 80.4). Histologic diagnosis was lacking in 25.2% of patients, D’Amico risk was high in 51.4%, and the median PSA at diagnosis was 19.00 ng/ml (IQR: 10.64, 46.60). Only 24.5% of the patients received a previous treatment with a curative intent ( Table 1 ). The median age at diagnosis was lower in this group of patients (61.6 years, IQR: 59.1, 68.5) than in those not receiving it (78.2 years, IQR: 74.0, 81.4) ( Table S1 ).
| Characteristics at diagnosis | |
|---|---|
| Age (years), median (IQR) n = 87 | 75.4 (67.8, 80.4) |
| PC diagnosis, n (%) | |
| Histological | 77 (74.8) |
| Clinical | 26 (25.2) |
| PSA at diagnosis (ng/ml), median (IQR) n = 89 | 19.00 (10.64, 46.60) |
| D’Amico risk at diagnosis, n (%) n = 70 | |
| Low | 10 (14.3) |
| Intermediate | 18 (25.7) |
| High | 36 (51.4) |
| Locally advanced | 6 (8.6) |
| N staging, n (%) n = 102 | |
| Nx | 66 (64.7) |
| N0 | 34 (33.3) |
| N1 | 2 (2.0) |
| Treatment with curative intent, n (%) n = 102 | |
| 0 | 77 (75.5) |
| 1 | 23 (22.5) |
| 2 b | 2 (2.0) |
| Salvage treatment after recurrence, n (%) n = 24 | |
| Yes | 2 (8.3) |
| No | 22 (91.7) |
| Characteristics at study inclusion | |
| Age (years), median (IQR) | 87.0 (81.0, 90.0) |
| Time from diagnosis to study inclusion (years), median (IQR) n = 85 | 9.32 (6.15, 14.07) |
| ECOG, n (%) n = 79 | |
| 0 | 25 (31.6) |
| 1 | 41 (51.9) |
| 2 | 10 (12.7) |
| Comorbidities | |
| No | 9 (8.7) |
| Yes | 94 (91.3) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree