Highlights
- •
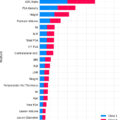
GGT for patients with PCa increased from 21 in Q2 2015 to 1,509 in Q3 2020.
- •
Proportion of GGT ordered by urologists increased from 0% in 2015 to 8.3% in 2020.
- •
Urology ordered more tests for patients under 70 years old.
- •
Urology ordered more tests for patients who reported negative family history.
Abstract
Purpose
The availability of targeted therapies for advanced prostate cancer led to the expansion of national guidelines recommending germline genetic testing. The aim of this study was to describe recent trends in germline test ordering patterns for patients with prostate cancer.
Materials and Methods
A retrospective cohort analysis of patients with prostate cancer who underwent germline testing through a single commercial laboratory (Invitae Corporation) between 2015–2020 was performed. Ordering trends between provider medical specialties were compared. Our primary hypothesis was that the proportion of tests ordered by urologists would increase over time.
Results
In total, 17,256 prostate cancer patients underwent germline genetic testing; 14,400 patients had an ordering provider with an associated medical specialty and were included in the final comparison cohort. Total prostate cancer patients undergoing germline testing increased quarterly from 21 in Q2 of 2015 to 1,509 in Q3 of 2020. The proportion of tests ordered by urologists increased from 0% in Q2 2015 to 8.3% in Q3 2020 ( P < 0.001). Compared to medical genetics, medical oncology, and other specialties, urology ordered more tests for patients under 70 years old (66% vs 51%–55%, P <0.004) and for patients who reported negative family history (25% vs 12%–20%, P = 0.012).
Conclusions
As awareness and indications for germline testing continue to expand, aggregate ordering volume is increasing, and urologists are becoming more involved in facilitating testing. This highlights the continued importance of educating urologists on the indications for and implications of germline genetic testing, as well as providing tools to support implementation.
1
Introduction
Prostate cancer (PCa) is the most commonly diagnosed visceral cancer in men [ ]. Pathogenic germline variants (PGVs) in PCa predisposition genes are found not only in metastatic disease, but also in high-risk localized disease, reported at 12% and 6% respectively, with genes such as BRCA2, CHEK2, BRCA1, ATM , and mismatch repair genes standing as some of the most commonly mutated [ , ]. In recent years, efforts to identify the incidence and type of PGVs in PCa were undertaken to enhance secondary cancer screening, cascade testing, prognosis, and susceptibility to novel therapeutics, such as poly ADP ribose polymerase (PARP) inhibitors [ , ]. The presence of germline mutations may also directly impact clinical trial eligibility, even in the localized setting, such as eligibility for neoadjuvant PARP inhibitor-based studies (ex. NCT05498272). Indeed, the National Comprehensive Cancer Network (NCCN) recommends germline genetic testing (GGT) for patients with high-risk localized and metastatic PCa [ ].
The identification of targetable genetic markers for certain aggressive PCa has caused a paradigm shift towards precision treatment [ ], which has highlighted the utility of GGT and expanded the guideline ordering indications [ ]. Many health systems are utilizing alternative service delivery models of GGT to adapt to the increased demand, often referred to as “mainstreaming” of GGT [ ].
This study aims to investigate GGT ordering trends in the recent shift toward precision medicine. We describe these trends across specialties over a recent 6-year span from a single GGT commercial laboratory and hypothesize that GGT is increasingly being ordered by urologists as indications expand, awareness increases, and test volume rises. Testing patient demographics are also described and compared across ordering specialties.
2
Materials and methods
2.1
Study design and patients
All patients with diagnosed PCa who underwent GGT between 03/27/2015 and 12/02/2020 through Invitae Corporation (San Francisco, California) and who consented to de-identified data sharing were included. Data were de-identified and collected from test requisition forms filled out by ordering clinicians. Each patient case included age at testing, race/ethnicity or ancestry information, test order date, ordering specialty, family history and number of genes ordered/analyzed. Cases with omitted data were not included in the comparison of that variable. Age at testing was stratified into <50 years old, 50–59, 60–69, and 70+. Racial or ethnic background was clinician-reported and categorized as White, African-American/Black, Asian, Ashkenazi Jewish, Hispanic, Mixed ethnicities, or Other. In this database, the “other” category did not specify the racial or ethnic groups included. Number of genes ordered in the testing panel were grouped as 1–20 genes, 21–40, 41–80, and 80+. Data was compared between ordering specialty cohorts.
2.2
Cohort definitions
Six cohorts were created based on available differentiating descriptors to group the cases by ordering provider specialty: medical genetics (including genetic counselors), medical oncology, urology, radiation oncology, primary care (physicians and APPs), other. “Other” includes every other ordering specialty that does not fit into 1 of the specified five groups. Cases without ordering provider information or vague descriptors were not grouped and therefore not included in the comparison analysis. While these 6 cohorts were used for the ordering trend analysis by year, when patient demographic data were compared, the cohorts were consolidated down to 4: medical genetics, medical oncology, urology, other. For clarity, the consolidated “other” cohort will be referred to as “Composite Other.”
2.3
Statistical analysis
Differences between cohorts were assessed by Pearson Chi-square test for categorical variables, and descriptive statistics were reported using tables, graphs, and charts. Post-hoc pairwise T-test was performed for comparing means. The primary outcome was the percentage of total tests each year ordered by Urology. Secondary outcomes included test ordering trends by specialty. Statistical analysis was performed using SPSS v.27 (IBM, Armonk, New York). Two-tailed P -value < 0.05 was considered statistically significant.
3
Results
3.1
Cohort patient demographics
17,256 PCa patients were included from 03/27/2015 to 12/02/20. Of the 14,400 patients assigned to an ordering provider cohort, 8,099 patients had tests ordered by medical oncology, followed by medical genetics (2,824), urology (915), primary care (581), and radiation oncology (438). The Other group had 1,543 patients, and 2,856 patients were unassigned. For cohort comparison, the Composite Other group (comprising primary care, radiation oncology, and Other), had 2,562 ( Table 1 ). Mean patient age at time of testing was youngest for the urology cohort (65.7 years). Clinician-reported race/ethnicity was primarily White (>72%) and most (>75%) of patients reported a positive family history of cancer ( Table 1 ).
| Variable | Medical Genetics | Medical Oncology | Urology | Other | P value |
|---|---|---|---|---|---|
| Mean Age (years ±SD) | 67.8 ± 9.1 | 68.7 ± 9.2 | 65.7 ± 9.0 | 67.9 ± 9.2 | < 0.001 |
| Age | |||||
| <50 | 86 (3.1%) | 177 (2.2%) | 44 (4.8%) | 79 (3.1%) | < 0.001 |
| 50–59 | 427 (15.2%) | 1098 (13.7%) | 171 (18.7%) | 349 (13.7%) | 0.002 |
| 60–69 | 1014 (36.2%) | 2886 (35.9%) | 383 (41.9%) | 975 (38.3%) | 0.012 |
| 70–79 | 1032 (36.8%) | 2928 (36.5%) | 268 (29.3%) | 918 (36.1%) | 0.001 |
| 80+ | 242 (8.6%) | 941 (11.7%) | 48 (5.3%) | 225 (8.8%) | 0.005 |
| Ethnicity/Race | |||||
| White (n, %) | 1996 (72.8%) | 6018 (77.9%) | 629 (78.9%) | 1946 (78.8%) | 0.003 |
| African-American/Black (n, %) | 295 (10.8%) | 710, (9.2%) | 84 (10.5%) | 149 (6.0%) | < 0.001 |
| Asian (n, %) | 52 (1.9%) | 120 (1.6%) | 3 (0.4%) | 32 (1.3%) | |
| Ashkenazi Jewish (n, %) | 94 (3.4%) | 220 (2.8%) | 26 (3.3%) | 95 (3.8%) | |
| Hispanic (n, %) | 87 (3.2%) | 235 (3.0%) | 26 (3.3%) | 50 (2.0%) | |
| Mixed (n, %) | 149 (5.4%) | 270 (3.5%) | 16 (2.0%) | 142 (5.7%) | |
| Other (n, %) | 70 (2.6%) | 157 (2.0%) | 13 (1.6%) | 56 (2.3%) | |
| Family History of Cancer | 0.012 | ||||
| Positive (n, %) | 2301 (83.9%) | 5908 (80.0%) | 561 (75.2%) | 2140 (88.4%) | |
| Negative (n, %) | 440 (16.1%) | 1478 (20.0%) | 185 (24.8%) | 280 (11.6%) | |
| Number of Genes on Ordered Panel | |||||
| 1–20 (n, %) | 711 (25.2%) | 2354 (29.1%) | 196 (21.4%) | 569 (22.2%) | < 0.001 |
| 21–40 (n, %) | 333 (11.8%) | 852 (10.5%) | 29 (3.2%) | 245 (9.6%) | < 0.001 |
| 41–80 (n, %) | 949 (33.6%) | 2341 (28.9%) | 223 (24.4%) | 996 (38.9%) | 0.024 |
| 80+ (n, %) | 831 (29.4) | 2552 (31.5%) | 467 (51%) | 752 (29.4%) | < 0.001 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree