Highlights
- •
Among individuals undergoing targeted biopsy of PI-RADS 5 lesions on prostate MRI, 15.2% were found to have Gleason Grade Group 1 prostate cancer.
- •
The majority of those managed with active surveillance (70.5%) experienced short term disease reclassification.
- •
Higher biopsy Decipher genomic classifier score was associated with biopsy Gleason reclassification among patients with PI-RADS 5 lesions and Gleason grade group 1 on initial biopsy.
- •
These findings support the role of confirmatory biopsy in patients with highly conspicuous prostate MRI findings and low-grade prostate cancer on initial biopsy.
Abstract
Background and Objective
As most Prostate Imaging Reporting and Data System (PI-RADS) 5 lesions on MRI harbor Gleason grade (GG) group ≥2 disease on biopsy, optimal management of patients with imaging-biopsy discordance remains unclear. To estimate grade misclassification, we evaluated the incidence of Gleason upgrading among patients with GG1 disease in the setting of a PI-RADS 5 lesion.
Methods
We conducted a single-institution retrospective analysis to identify patients with GG1 prostate cancer on fusion biopsy with MRI demonstrating ≥1 PI-RADS 5 lesion. Primary study outcome was identification of ≥GG2 disease on subsequent active surveillance (AS) biopsy or radical prostatectomy (RP). We used multivariable models to examine factors associated with reclassification.
Results
We identified 110 patients with GG1 disease on initial biopsy and ≥1 PI-RADS 5 lesion. There were 104 patients (94.6%) initially managed with AS and 6 (5.5%) received treatment. Sixty-one patients (58.7%) on AS underwent additional biopsies. Of these, 43 (70.5%) patients had tumor upgrading, with 32 (74.4%) upgraded on first surveillance biopsy. Forty-four (40%) patients ultimately received treatment, including prostatectomy in 15 (13.6%) and radiation in 25 (22.7%). Two patients (1.8%) developed metastases. In multivariable models, genomic classifier score was associated with upgrading. Limitations include a lack of multi-institutional data and long-term outcomes data.
Conclusions
Most patients diagnosed with GG1 prostate cancer on MRI-Ultrasound fusion biopsy in the setting of a PI-RADS 5 lesion were found to have ≥GG2 disease on subsequent tissue sampling, suggesting substantial initial misclassification and reinforcing the need for confirmatory testing.
1
Introduction
Active surveillance (AS), a period of close disease monitoring, is the recommended management strategy for most patients with low-grade (Gleason grade group (GG) 1) prostate cancer. The safety of AS has been supported by level-1 evidence demonstrating equivalent 15-year overall survival compared with immediate definitive treatment [ ]. However, a meaningful subset of patients initially enrolled in AS experience disease reclassification due to changes in cancer grade, stage, or PSA. Early upgrade on follow-up biopsy or prostatectomy is commonly attributed to initial disease misclassification, highlighting the potential risk for biopsy under-sampling. To enhance selection for AS, a host of clinical prediction tools, genomic biomarkers, and imaging modalities have been developed to estimate disease stage and cancer aggressiveness [ ].
In the setting of rapid adoption, prostate magnetic resonance imaging (MRI) has been shown to improve the accuracy of initial prostate cancer detection, refine grade concordance [ ], and aid in upfront treatment selection [ ]. Discordance between prostate MRI and biopsy findings raises concern for misclassification on imaging or mistargeting during biopsy, however the optimal clinical management in this scenario is unclear. This is particularly relevant for those with highly suspicious MRI findings, such as Prostate Imaging Reporting & Data System (PI-RADS) 5 lesions but negative or GG1 prostate cancer on diagnostic biopsy, given the high positive predictive value (PPV) of PI-RADS 5 for the detection of clinically significant cancer [ ]. Prior studies have also observed associations between MRI findings and disease outcome, suggesting that MRI may serve as a staging and diagnostic tool as well as a prognostic biomarker. [ ] In parallel, tissue-based gene expression testing using commercial assays associated with prostate cancer outcome have become increasingly used in clinical practice, and may prognostic estimates relevant to active surveillance reclassification [ , , ]. However, current AS clinical practice guidelines do not explicitly include PI-RADS findings as an independent criterion for decision-making [ ]. In addition, it is not known whether clinical or genomic information can identify which patients with PI-RADS 5 lesions are more or less likely to reclassify in the short term.
We aimed to evaluate early grade reclassification among patients with GG1 prostate cancer on initial biopsy in the setting of PI-RADS 5 findings on prostate MRI and identify factors associated with its occurrence. Given low national use of confirmatory biopsy testing and high attrition from AS protocols we further sought to evaluate the use of follow-up biopsy among those managed with AS [ ]. We hypothesized that among patients with GG1 prostate cancer on biopsy in the setting of a PI-RADS 5 lesion, a substantial proportion would experience disease reclassification – either at follow-up biopsy or radical prostatectomy (RP), owing to initial disease under-grading on their diagnostic biopsy.
2
Materials (patients) and methods
2.1
Study design and patient selection
We conducted a retrospective cohort study of patients diagnosed with International Society of Urological Pathology (ISUP) GG1 prostate cancer from October 2013 to December 2022 whose MRI nearest to diagnosis revealed ≥1 lesion classified as PI-RADS 5. Yale Institutional Review Board approval for chart review was obtained (IRB number 0805003787). Active surveillance was defined as the absence of treatment within 1 year of initial diagnosis and intended management with AS noted in clinical documentation. Patients were considered lost to follow-up and were excluded if there was no follow-up appointment or procedure documented after initial biopsy and were censored at the date of last follow-up.
The institutional active surveillance protocol strongly recommended a repeat MRI and confirmatory prostate biopsy within 12 months of initial diagnosis. The protocol consisted of regular PSA monitoring, yearly or every-other-year MRI and biopsy, depending on individual risk, life expectancy, and patient preference. Genomic testing was commonly used but not required among patients electing initial active surveillance.
We evaluated the natural history of patients following diagnosis. Among patients managed with AS or RP, we compiled pathology findings from follow-up biopsies and/or surgical specimen. The primary study outcome was Gleason reclassification to Gleason Grade ≥2 prostate cancer on a follow-up prostate biopsy or RP. The secondary outcomes included conversion from AS to active treatment, receipt of a follow-up biopsy, and loss to follow-up.
2.2
Multiparametric MRI
Multiparametric prostate MRI was centrally performed at one multi-site institution and the studies were reviewed by radiologists using the PI-RADS v2 and v2.1 scoring system. Of note, criteria for PI-RADS 5 lesions did not change between PI-RADS v2 and v2.1. All MRI studies were performed using a 3-tesla system and a pelvic phased-array coil. Images included multiplanar T2-weighted images, axial diffusion-weighted imaging (b-values = 50 s/mm 2 and 1000 s/mm 2 ), and dynamic gadolinium contrast-enhanced imaging.
2.3
MRI-ultrasound fusion-targeted biopsy
All patients received prostate MRI prior to initial biopsy. Prostate biopsies were performed in an MRI-Ultrasound fusion approach using either the Artemis (Eigen Health) or ExactVu (Exact Imaging) biopsy platform. Lesions with features suspicious for prostate cancer on imaging were outlined on T2-weighted MRI sequences, and computer-assisted segmentation was performed. Transrectal 12-core template systematic and targeted biopsies were obtained on biopsy sites identified by the Artemis or ExactVu system. A single core was obtained from each of the 12 systematic sites. As part of our institutional protocol, a total of 5 cores per lesion were recommended at the time of targeted biopsy.
2.4
Statistical analysis
We compiled the clinical, demographic, and radiologic features of the study cohort. These included patient age at diagnosis, race/ethnicity, digital rectum examination results (normal or abnormal), serum PSA concentration, PSA density (PSA divided by MRI-determined prostate volume), Decipher genomic classifier (GC) score modeled per 0.1-unit increase (among patients who received testing), number of PI-RADS 5 lesions, number of prostate MRI-visible lesions, MRI lesion volume, prostate volume, time on AS, and number of biopsy sessions undergone. For patients who received multiple genomic tests, only the first result following diagnosis was included. Among patients initially managed with AS, we calculated the proportion who received follow-up biopsy and who eventually received definitive treatment. We then calculated the proportion of patients who experienced Gleason reclassification on subsequent tissue sampling.
Next, we examined the associations between clinical and pathological characteristics and subsequent Gleason upgrading using univariable and multivariable logistic regression modeling. Multivariable models were adjusted for all clinical, demographic, genomic, and radiologic variables collected. Using a similar approach, we constructed models to evaluate the factors associated with treatment after initial AS. Upgrading and receipt of treatment were defined as binary variables (yes/no) for each patient, with patients not experiencing upgrading or treatment serving as modeling control groups. Statistical analyses were conducted using R version 4.3.1. All tests were 2-sided and a P -value threshold of < 0.05 was used to define statistical significance.
3
Results
We identified 110 patients undergoing MRI ultrasound fusion biopsy biopsy between October 2013 and December 2022 who had GG1 disease on initial biopsy and PI-RADS 5 findings on prostate MRI. Among all patients at our institution with GG1 disease on initial biopsy ( n = 911), there were 117 (12.8%) with PI-RADS 5 lesions. Among all patients with ≥1 PI-RADS 5 lesion undergoing biopsy ( n = 770), there were 117 patients (15.2%) with highest GG1 on prostate biopsy ( Figure 1 ). We identified 7 patients with GG1 disease and PI-RADS 5 lesions who had no follow-up data beyond their initial biopsy, and we excluded them from further analysis.
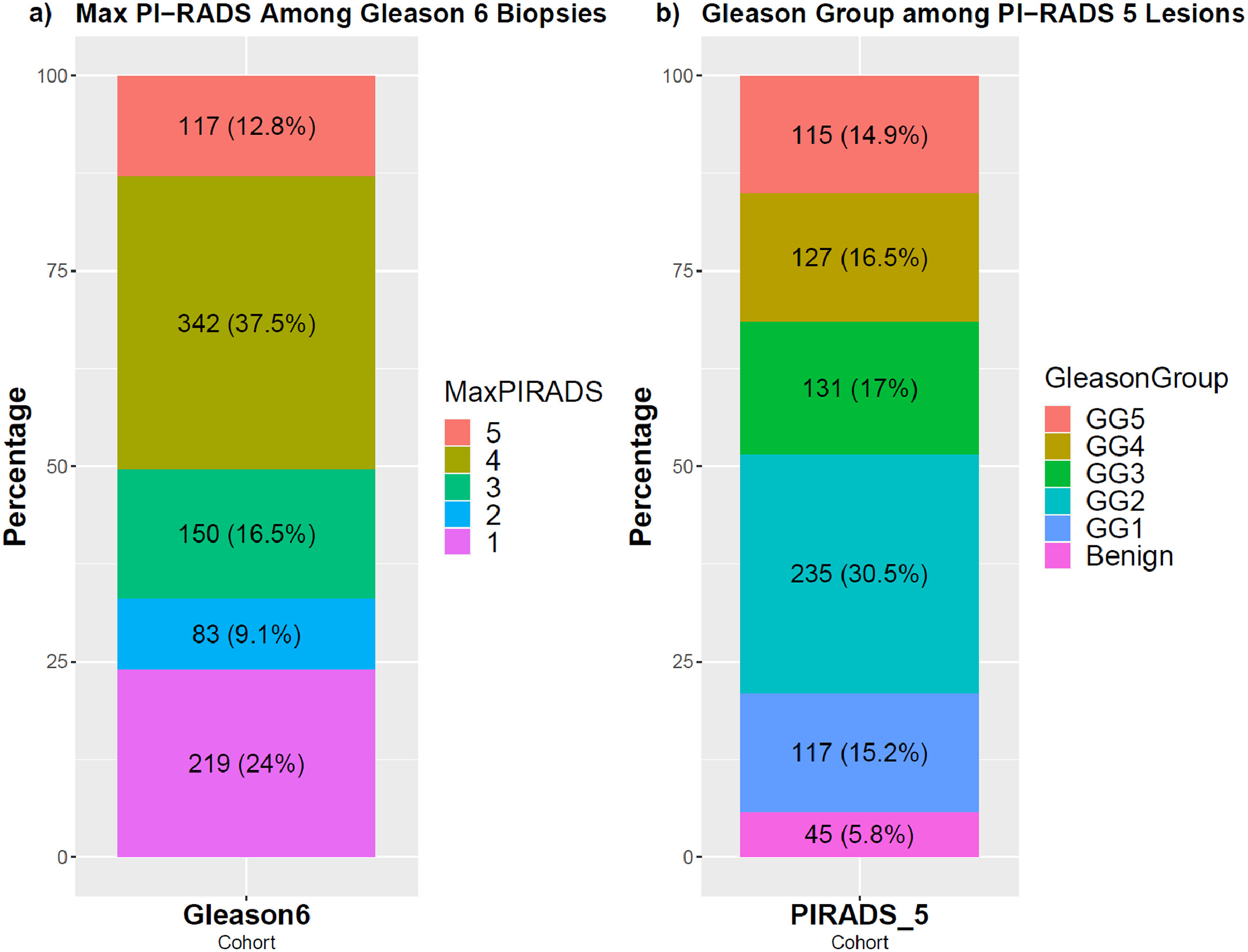
Of the 110 patients with both GG1 disease and PI-RADS 5 lesions on MRI, the mean age at diagnosis was 65.5 years and median follow-up time was 5.1 years (Interquartile range (IQR): ± 3.6 years). The median PSA concentration and PSA density at diagnosis were 6.45 ng/mL (IQR ± 4.65 ng/mL) and 0.14 ng/mL/cc (IQR ± 0.08 ng/mL/cc) respectively. There were 15 patients (13.6%) with >1 PI-RADS 5 lesion. In addition to 12 systematic biopsy cores, each patient had an average of 8.3 targeted cores per biopsy session taken from MRI regions of interest (average of 20.3 total cores per patient per biopsy session). The mean number of targeted biopsy cores taken per region of interest was 4.9. Following diagnosis, 104 patients (94.6%) initiated AS, 3 underwent prostatectomy, 2 underwent radiation therapy (RT), and 1 was started on androgen deprivation therapy (ADT) ( Table 1 ). A total of 87 (79.1%) patients received genomic classifier testing following diagnosis with a median Decipher score of 0.41 (IQR ± 0.26).
| Cohort Description | Total |
|---|---|
| Gleason 6 PI-RADS 5 Patient Characteristics | |
| Total Patients | 110 |
| Deceased | 4 (3.6%) |
| Age (mean ± SD) | 69.58 ± 7.34 years |
| Age at Diagnosis (mean ± SD) | 65.42 ± 7.34 years |
| Race | |
| White | 94 |
| Black | 11 |
| Hispanic | 5 |
| # of Biopsies Received | |
| 1 | 49 |
| 2 | 34 |
| ≥3 | 27 |
| Clinical Characteristics | |
| PSA at Diagnosis (median ± IQR) | 6.45 ± 4.65 ng/mL |
| MRI Prostate Volume (median ± IQR) | 52.55 ± 32.39 mL |
| PSA Density | 0.138 ± 0.08 ng/mL/cc |
| Gleason Score at Diagnosis | 3+3 |
| Max PI-RADS Score | 5 |
| # of PI-RADS 5 Lesions | |
| 1 | 95 (86.3%) |
| >1 | 15 (13.6%) |
| Treatment | |
| Following Diagnosis | |
| Prostatectomy | 3 |
| Radiation w/ ADT | 2 |
| ADT only | 1 |
| Following Active Surveillance | |
| Prostatectomy | 12 |
| Radiation w/ ADT | 11 |
| Radiation w/o ADT | 12 |
| ADT only | 2 |
| Other (Cryoablation/Nanoknife) | 4 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree