Highlights
- •
Initial trial results examining the efficacy of adjuvant immune checkpoint inhibitors in the setting of high-risk, resected muscle invasive urothelial carcinoma provided disparate results.
- •
In this systematic review and meta-analysis of 3 clinical trials involving 2,220 patients with muscle invasive urothelial carcinoma, adjuvant immune checkpoint inhibitors were associated with a significant benefit in disease-free survival.
- •
Patients with muscle invasive urothelial carcinoma are more likely to benefit from adjuvant immunotherapy compared to observation/placebo.
Abstract
Despite surgical resection, many patients with muscle invasive urothelial carcinoma (MIUC) experience recurrence. Adjuvant immune checkpoint inhibition (ICI) following radical resection in patients with MIUC demonstrates disparate outcomes among phase III randomized controlled trials (RCTs). Our objective was to synthesize available data regarding the disease-free survival (DFS) benefit of adjuvant ICIs for patients with MIUC and evaluate the overall safety profile of ICIs in this setting. The protocol was registered with PROSPERO, CRD42022352587. We searched MEDLINE, Embase, CENTRAL, and relevant conference proceedings from inception up to January 29, 2024. Only phase III RCTs comparing adjuvant ICI versus placebo/observation were selected. Study screening and selection, along with data extraction was performed in duplicate according to a predefined registered protocol. The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline was used. Quality assessment was performed using the Cochrane risk-of-bias (RoB 2) tool for randomized trials. The primary and secondary endpoints were DFS and serious adverse events, respectively. All outcomes were analyzed using random-effects meta-analysis owing to inter-study heterogeneity. Sensitivity and subgroup analyses were performed to identify potential sources of heterogeneity. A priori defined subgroups of interest included positive program death-ligand 1 (PD-L1) expression, previous use of neoadjuvant chemotherapy (NAC), primary tumor origin, pathologic lymph node status, and baseline Eastern Cooperative Oncology Group performance status. Pooled results across the 3 RCTs (2,220 patients) demonstrated significantly improved DFS for patients treated with ICI in the intention-to-treat cohorts (HR 0.76, 95% CI 0.65-0.90). There was considerable clinical and statistical heterogeneity (I 2 = 44%) due to differences in inclusion criteria and interventions. Overall, there was a low risk of bias among the RCTs. Regarding subgroup analyses, there was significant benefit among patients with negative PD-L1 expression (HR 0.76, 95% CI 0.64-0.90), those who received prior NAC (HR 0.69, 95% CI 0.52-0.91), and patients with lower tract (HR 0.71, 95% CI 0.55-0.92) but not upper tract disease (HR 1.21, 95% CI 0.87-1.68). This pooled analysis of DFS and safety provides support for ICI utilization in the setting of high-risk resected MIUC.
1
Introduction
A standard of care for muscle-invasive urothelial carcinoma (MIUC) is neoadjuvant cisplatin-based chemotherapy (NAC) in eligible patients followed by radical resection [ , ]. Approximately 50% of patients subsequently develop recurrence following radical surgery [ ]. There is a significant unmet need in cisplatin-ineligible MIUC patients for novel systemic therapies. Phase III randomized controlled trials (RCTs) examining adjuvant immune checkpoint inhibitors (ICI) targeting programmed cell death–1 (PD-1) and its ligand PD-L1 have been examined with conflicting results [ , ]. In light of extended follow up of CheckMate-274 and recent results from the AMBASSADOR trial [ , ], we performed a systematic review to assess the summative disease free survival (DFS) benefit, adverse event profile, and examine potential subgroups of clinical interest.
2
Materials and Methods
We performed a systematic review according to the to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [ ]. The protocol was registered with PROSPERO, CRD42022352587.
2.1
Search strategy and study selection
Using terms “adjuvant” and “immunotherapy” and “urothelial carcinoma”, we searched MEDLINE, Embase, CENTRAL, and relevant conference proceedings (European Society for Medical Oncology and American Society of Clinical Oncology meetings) from inception up to January 29, 2024. The population was patients with MIUC. The outcomes of interest were survival and safety. The intervention was adjuvant ICI. The comparison was placebo/observation. Using Covidence, the records were screened by 2 independent reviewers (L.O.T. and C.R.) and disagreements were resolved by a third reviewer (R.S.). We included only English-language phase III randomized controlled trials (RCTs). Nonrandomized trials and retrospective studies were excluded.
2.2
Endpoints and quality assessment
The primary and secondary endpoints were DFS and serious adverse events (sAEs: AEs ≥ grade 3), respectively. Quality assessment was performed using the Cochrane risk-of-bias (RoB 2) tool for randomized trials [ ].
2.3
Data extraction and statistical analysis
Data were also extracted in duplicate. For DFS, hazard ratios (HRs) with their corresponding 95% confidence intervals (CIs) were abstracted from the selected studies. Using an inverse variance approach, HRs were pooled using logarithmic transformation. For sAEs, event rates were pooled using the Mantel-Haenszel approach and effect sizes were expressed as odds ratios (ORs) with 95% CIs. We performed a priori defined subgroup (i-vi) and sensitivity (vii) analyses: (i) PD-L1 expression; (ii) previous use of NAC; (iii) origin of primary tumor (upper vs. lower urinary tract); (iv) pathologic lymph node (pN) status; (v) baseline Eastern Cooperative Oncology Group performance status (ECOG PS); and (vii) exclusion of CheckMate-274 (as it compared ICI to placebo, instead of observation). Using Review Manager 5.4 (The Cochrane Collaboration, Copenhagen, Denmark), we performed all meta-analyses with random-effects models owing to inter-study heterogeneity. Heterogeneity was assessed using the I 2 statistic. P values were 2-sided and values <0.05 were deemed significant.
3
Results
3.1
Study characteristics and quality
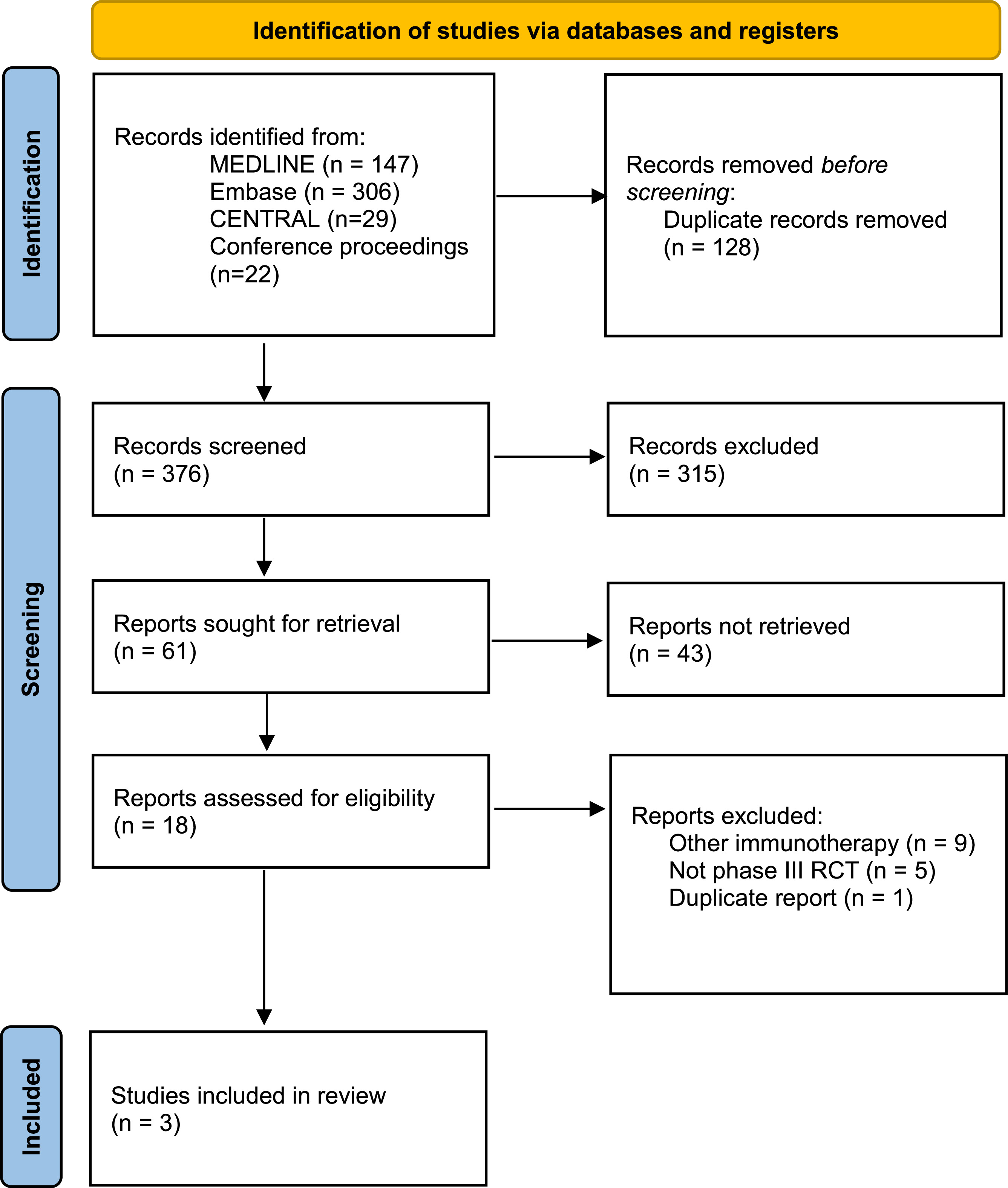
Three phase III RCTs with a total of 2,220 patients were included ( Fig. 1 and Table 1 ). All studies included DFS as the primary outcome, with treatment duration being 12 months. Inclusion of upper tract MIUC patients ranged between 5 and 20%. All studies included patients who had received NAC but had persistent high-risk features (ypT2-4a or ypN+/positive margins) or those that did not receive NAC and refused or were ineligible for adjuvant chemotherapy (pT3-4a or pN+/positive margins). There were notable differences between the studies of interest, specifically heterogeneity in the control arm, with either placebo (CheckMate-274) or observation (IMVigor010 and AMBASSADOR) ( Table 1 ). In the intention to treat analyses, 2 studies met the primary endpoint of improvement in DFS (CheckMate-274 and AMBASSADOR) while one did not (IMVigor010). Overall, there was a low risk of bias among the RCTs ( Fig. 2 ).
| Study | Year of publication | Histology | Arms | Participants (n) | Median age (years) | Female sex (%) | PD-L1 positive (%) | TPS/CPS score cut-off, assay for PDL1 | Previous NAC (%) | Primary tumor site (%) | pN+ (%) | Median follow-up (months) | Primary endpoint | Secondary endpoints |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IMvigor 010 | 2021 | MIUC | Atezolizumab | 406 | 67 | 21 | 48 | CPS ≥5%, VENTANA IHC SP142 | 48 | LUT: 93; UUT: 7 | 52 | 21.9 | DFS (ITT) | OS, DSS, DMFS, NUTRFS, PK, PROs, safety |
| Observation | 403 | 66 | 22 | 49 | 47 | LUT: 94; UUT: 6 | 52 | |||||||
| CheckMate 274 | 2021 | MIUC | Nivolumab | 353 | 65.3 (mean) | 24.9 | 39.7 | TPS ≥1%, Dako IHC 28-8 pharmDx | 43.4 | LUT: 79; UUT: 21 | 47.3 | 31.6 b | DFS (ITT & PD-L1 ≥1%) | OS, DSS, NUTFRS, DMFS, PROs, safety |
| Placebo | 356 | 65.9 (mean) | 22.8 | 39.9 | 43.5 | LUT: 78.9; UUT: 21.1 | 47.2 | |||||||
| AMBASSADOR | 2024 a | MIUC | Pembrolizumab | 354 | 69 | 23.4 | 57.1 | CPS ≥10%, Dako IHC 22c3 pharmDx | 65.3 | LUT: 77.1; UUT: 22.9 | 50.9 | 22.3 | DFS & OS (ITT) | DFS/OS based on PD-L1 status, Safety |
| Observation | 348 | 68 | 27.3 | 57.8 | 62.6 | LUT: 79.3; UUT: 20.7 | 48.8 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree