Highlight
- •
Neoadjuvant therapy significantly improved DFS and OS in RCC patients with IVC thrombus.
- •
Patients receiving neoadjuvant therapy had a 2-year recurrence-free survival of 70.9%.
- •
Pathological complete remission with no viable tumor cells was observed in 10.5% of cases.
- •
The study highlights the mid- to long-term benefits of neoadjuvant systemic therapy.
Abstract
Background
The standard treatment for non-metastatic renal cell carcinoma (RCC) with inferior vena cava (IVC) thrombus is complete surgical resection; however, this procedure is complex and carries high complication rates and perioperative mortality. Previous studies have explored preoperative multimodal therapy to reduce surgical difficulty, but limited evidence prevents guideline recommendations. This study aimed to investigate the impact of neoadjuvant therapy on the prognosis of patients with RCC and IVC thrombus without distant metastasis.
Methods
Data from 2006 to 2024 on RCC patients with IVC thrombus undergoing radical nephrectomy plus IVC thrombus resection were collected. Patients received neoadjuvant therapy, including tyrosine kinase inhibitors or immune checkpoint inhibitors, followed by surgery. Tumor size and thrombus height were assessed by computed tomography. Disease-free survival (DFS) and overall survival (OS) were calculated using Kaplan-Meier curves. Multivariate analysis was used to identify factors predicting DFS.
Results
Thirty-one patients who did not receive neoadjuvant chemotherapy therapy (NAC-Naive group) and 19 patients who received neoadjuvant chemotherapy therapy (NAC group) were analyzed. The NAC group showed significant reductions in primary renal tumor size and neutrophil-to-lymphocyte ratio compared to the NAC-Naive group just before nephrectomy. The NAC group had significantly improved DFS and OS. Median DFS and OS were not reached in the NAC group compared to 26.3 months and 73.5 months, respectively, in the NAC-Naive group. The NAC group had a 2-year recurrence-free survival rate of 70.9% compared to 50.6% in the NAC-Naive group. Multivariate analysis identified a preoperative tumor size of 10 cm or larger and lack of neoadjuvant therapy as poor prognostic factors for DFS.
Conclusion
Neoadjuvant therapy significantly improves the prognosis of RCC patients with IVC thrombus. This therapy reduces surgical invasiveness and has a mid- to long-term preventive effect on recurrence. These findings support the potential benefit of neoadjuvant systemic therapy in improving outcomes for this patient population.
1
Introduction
Renal cell carcinoma (RCC) accounts for 3% of solid tumors, and it is estimated that 4%–10% of RCC cases involve inferior vena cava (IVC) thrombus [ ]. The standard treatment is complete surgical resection, particularly for RCC with IVC thrombus that has not metastasized. However, this surgery is complex and challenging, with high surgical invasiveness, complication rates, and perioperative mortality [ ].
Clinical trials [ , ] and retrospective studies [ , ] have investigated the potential benefit of preoperative multimodal therapy to reduce surgical difficulty. Previous studies have reported the feasibility of minimally invasive surgery due to thrombus reduction; however, the rarity of the condition and limited evidence have prevented guidelines from recommending neoadjuvant treatment [ , ].
We have previously reported on the utility of neoadjuvant therapy for advanced RCC with IVC thrombus, focusing on the reduction of surgical invasiveness and tumor size [ , , ]. In this study, we retrospectively examined the impact of presurgical neoadjuvant on the prognosis of patients with IVC tumor thrombus who do not have distant metastasis.
2
Methods
From 2006 to 2024, we collected data on RCC patients with IVC thrombus who underwent radical nephrectomy plus IVC thrombus resection in Kobe University Graduate School of Medicine. This study adhered to the principles of the Declaration of Helsinki and was approved by the Medical Ethics Committee of Kobe University Graduate School of Medicine (No. B240116). Patient consent was obtained via an opt-out option on the website. All patients underwent contrast-enhanced chest and abdominal computed tomography and bone scintigraphy at the initial consultation. Patients with regional lymph node metastasis or ipsilateral adrenal gland metastasis were included in the study, but patients with other distant metastases were excluded.
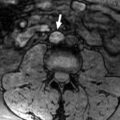
Patients who received neoadjuvant therapy underwent percutaneous renal biopsy, followed by a planned neoadjuvant treatment period of approximately three months. The duration was adjusted by the attending physician based on the patient’s overall condition and disease progression. The patients’ treatment choices were clearly divided by time periods. Notably, cases before 2015 did not receive neoadjuvant therapy. From 2016 to 2019, the standard treatment for patients receiving pazopanib monotherapy was tyrosine kinase inhibitor (TKI) treatment. Patients diagnosed from 2020 onward were administered avelumab plus axitinib or pembrolizumab plus lenvatinib. A washout period was observed for TKIs based on their elimination half-lives before radical surgery. Tumor size was measured as the maximum diameter of the renal tumor, excluding the thrombus, on computed tomography. The height of the thrombus was classified according to the MAYO classification [ ]. Pathological findings of the nephrectomy specimens were diagnosed by genitourinary pathologists who were informed of the patient’s details.
The surgical procedure was primarily open surgery. The tumor and IVC thrombus were removed en bloc. For cases where the tumor extended to the atrium, cardiopulmonary bypass was used for resection. In patients whose thrombus was reduced to the renal vein due to neoadjuvant therapy, surgery was performed laparoscopically or with robotic assistance.
Statistical analysis was performed using R software. Kaplan-Meier curves were created for disease-free survival (DFS), defined as the period from surgery to recurrence or death, and overall survival (OS), defined as the period from surgery to death. Additionally, for patients who survived without recurrence for 6 months post-surgery, DFS was calculated from the 6-month mark to the date of recurrence or last follow-up. To identify factors predicting DFS, multivariate analysis was conducted using stepwise Cox proportional hazards model. The variables included American Society of Anesthesiologists Physical Status (ASAPS) score of 1, preoperative modified Glasgow prognostic score [ ] of 0, preoperative neutrophil-to-lymphocyte ratio (NLR) of 3 or higher, presence of NAC, preoperative renal tumor size, tumor laterality, preoperative MAYO classification of 3 or higher, sex, histological type, and age 75 or older.
3
Results
The NAC-Naive group consisted of 31 patients who underwent nephrectomy and IVC thrombus resection without neoadjuvant therapy. Among patients with RCC with IVC thrombus and no distant metastasis, 21 were identified who received combination therapy with either TKI alone or immune checkpoint inhibitor combination therapies. Of these 21 patients, we excluded 2 patients (1 who received pazopanib, and 1 who received avelumab plus axitinib but could not undergo radical surgery). The NAC group consisted of 19 patients. The median primary renal tumor size was approximately 90 mm, with 11 patients showing lymph node enlargement and 3 patients having adrenal metastasis in the entire cohort. No significant differences were observed between the NAC-Naive group and the NAC group at initial diagnosis ( Supplementary Table 1 ). In the NAC-Naive group, over a median observation period of 63.4 months (range: 1.0–184.3), 23 recurrences and 16 deaths were noted. The sites of recurrence in the NAC-Naive group, including overlapping cases, were the lungs in 12 cases, liver in 3 cases, lymph nodes in 3 cases, bones in 2 cases, within the IVC in 2 cases, local recurrence in 1 case, pulmonary tumor embolism in 1 case, and other sites in 6 cases. In the NAC group, over a median observation period of 42.4 months (range: 3.5–93.7) postsurgery, nine recurrences and one death were observed. The recurrence sites in the NAC group were the lungs in 3 cases, bones in 1 case, and local recurrence in 1 case.
We found a difference in ASAPS score due to hypertension, a side effect of TKI, between the NAC-Naive group and the NAC group before radical surgery. In the NAC group, there was a significant reduction in the median size of the primary renal tumor (to 62 mm) and the median NLR (to 2.54) compared to the NAC-Naive group. In the NAC group, tumor thrombus reduction based on the MAYO classification was observed in 10 patients (52.6%). Among these patients, one patient had a level 1 tumor thrombus reduced to the renal vein. Of the 6 level 2 patients, one had tumor thrombus reduced to the renal vein, and one reduced to level 1. Among the seven level 3 patients, four had tumor thrombus reduced to level 2. Of the 5 level 4 patients, 1 had tumor thrombus reduced to level 1 and 3 reduced to level 2 ( Table 1 ). The median tumor length from the cranial side of the renal vein inflow to the tip of the inferior vena cava tumor thrombus was 75 mm (range: 7–138 mm) before treatment, and it was reduced to 30 mm (range: 0–132 mm) after treatment. In the NAC-Naive group, 2 patients received interferon therapy and two received axitinib therapy as adjuvant treatments. In the NAC group, 10 patients received pazopanib, eight received avelumab plus axitinib, and 1 received pembrolizumab plus lenvatinib as neoadjuvant therapy ( Supplementary Table 2 ). No patients continued adjuvant treatment for more than 1 year.
| Characteristics | Group | NAC-Naive group before radical nephrectomy (N=31) | NAC group before radical nephrectomy (N=19) | P (vs. NAC-Naive) |
|---|---|---|---|---|
| Age, median (range) | 71(44–84) | 68(28–81) | 0.719 | |
| Sex (%) | Male | 24 (77.4) | 10 (52.6) | 0.117 |
| Female | 7 (22.6) | 9 (47.4) | ||
| Side (%) | Right | 25(80.6) | 18(94.7) | 0.229 |
| Left | 6(19.4) | 1(5.3) | ||
| ASA-score (%) | 1 | 13(41.9) | 2(10.5) | 0.028 * |
| 2 | 17 (54.8) | 16(84.2) | ||
| 3 | 1(3.2) | 1(5.3) | ||
| Tumor size, median (mm, range) | 89(43–124) | 62(20–135) | 0.031 * | |
| Mayo classification (%) | 1 | 8(25.8) | 2(10.5) | 0.28 |
| 2 | 9(29) | 8(42.1) | ||
| 3 | 9(29) | 5(26.3) | ||
| 4 | 5(16.1) | 2(10.5) | ||
| Tumor reduced to within the renal vein | 0 | 2(10.5) | ||
| Length of the inferior vena cava tumor thrombus (mm, range) + | 45(0–151) | 30(0–132) | 0.548 | |
| Clinical N (%) | 0 | 27(87.1) | 12(63.2) | 0.312 |
| 1 | 4(12.9) | 7(36.8) | ||
| Ipsilateral adrenal metastasis (%) | 2(6.5) | 1(5.3) | 1 | |
| NLR (range) | 3.14(1.27–6.68) | 2.54(1.53–4.72) | 0.037 * | |
| mGPS (%) | 0 | 20(64.5) | 9(47.4) | 0.371 |
| 1 | 4(12.9) | 5(26.3) | ||
| 2 | 7(22.6) | 5(26.3) |
⁎ significant. NLR: neutrophil to lymphocyte ratio, mGPS: modified Glasgow prognostic score.
The median intraoperative blood loss was 1820 cc (range: 250–11346) in the NAC-Naive group, compared to 555 cc (range: minimal–6945) in the NAC group, with a significantly lower amount observed in the NAC group ( P = 0.010). The median operative time was 383 minutes (range: 210–904) in the NAC-Naive group, compared to 419 minutes (range: 120–729) in the NAC group, with no significant difference between the groups ( P = 0.905).
Pathological examination of resected tissue showed 2 cases of pathological complete remission in the NAC group. No lymph node metastasis was observed in the NAC group; however, 2 cases had necrosis without viable tumor cells in the lymph nodes. Both patients who achieved pathologic complete response were treated with the combination of avelumab and axitinib. Regarding the thrombus response, 1 patient’s thrombus reduced from Mayo level 4 to level 1, while the other patient’s thrombus reduced from level 3 to level 1. The ISUP grade was higher in the NAC group; however, the difference was not significant. In the NAC group, 18 cases (94.7%) showed a high rate of necrosis within the tumor ( Table 2 ).