Highlights
- •
A prospective study to validate the diagnostic performance of CRiSS in SCC penis.
- •
CRiSS can avoid ILND with a zero false negative rate, irrespective of cN status.
- •
CRiSS can identify candidates for ILND after a negative FNAC and biopsy of cN+ nodes.
- •
It can identify patients for concomitant penectomy and ILND.
- •
MRI is a suitable replacement for ultrasonography if not standard of care (CRiSS-M).
Abstract
Objective
To prospectively validate the diagnostic performance of C lino-radio-pathological Ri sk S coring S ystem (CRiSS) for prediction of inguinal lymph-node metastasis (ILNM) in squamous cell carcinoma of penis.
Materials and Methods
A prospective observational study of all patients with SCC penis was conducted between January 1, 2021, and December 31, 2023, at our institute. Data regarding all CRiSS parameters and MRI features of >8mm size and presence of necrosis or irregular outline were recorded, and patients were assigned CRiSS scores and groups. All included patients were subjected to primary surgery (partial/total penectomy) along with bilateral radical inguinal lymph-node dissection. Multivariate logistic regression analysis was performed with both USG and MRI. Sensitivity and specificity were calculated for CRiSS scores and groups.
Results
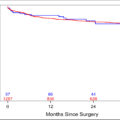
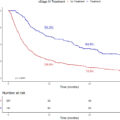
A total of 86 patients were enrolled during the study period. Size of lymph-node greater than 8mm (HR: 4.502) and irregular outline or presence of necrosis (HR: 4.002) in MRI were significantly associated with ILNM along with all other CRiSS variables in multivariate analysis. CRiSS groups had a sensitivity of 100% and a specificity of 85.71%. CRiSS could diagnose ILNM with a sensitivity of 100% in both palpable and non-palpable groins.
Conclusions
CRiSS can identify patients in whom ILND can be avoided with a zero false negative rate, irrespective of clinical lymph-node status. CRiSS can identify the patients who are candidates for ILND even after a negative FNAC and biopsy of palpable lymph-nodes. It can identify patients for concomitant penectomy and ILND. MRI is a suitable replacement for ultrasonography if not standard of care (CRiSS-M).
1
Introduction
Penile carcinoma is a rare neoplasm. Its incidence varies from 1/100000 in developed countries to 2-4/100000 in developing countries [ ]. Inguinal lymph-node metastasis (ILNM) carries a poor prognosis [ ]. Inguinal lymph-node dissection (ILND) improves survival, especially in cases of low lymph-node burden [ ]; however, the procedure is associated with significant morbidity [ ]. This underlines the importance of accurately predicting ILNM to balance the risk of morbidity associated with ILND as compared to its expected benefits. Current guidelines recommend ILND as a staged procedure after primary surgery in clinically N0 cases if high-risk features in the form of >T1 and lympho-vascular invasion (LVI) are present. If lymph-nodes are palpable, ILND is indicated if proven positive by biopsy [ , ]. With the paucity of high-level evidence, ideal indications for upfront ILND are still a controversy.
We devised a C lino-radio-pathological Ri sk S coring S ystem (CRiSS) to predict ILNM in carcinoma penis in 2020 [ ]. Based on a multivariate logistic analysis of retrospective data, 8 significant predictors were derived, and scores (0, 1, or 2) were assigned. A total score of 0 to 9 was calculated for each patient. Patients with scores of 0, 1, and 2 were grouped as low risk; scores of 3 and 4 were grouped as intermediate risk; and scores of 5, 6, 7, 8, and 9 were grouped as high risk ( Table 1 ). The incidence of ILNM was 0%, 41.4%, and 89.5%, respectively, for the low, intermediate, and high-risk groups, respectively.
| Parameters | 0 points | 1 point | 2 points | CRiSS score (USG) | CRiSS-M score (MRI) | |
|---|---|---|---|---|---|---|
| Clinical | Size of primary | <3cm | >/=3cm | 0/1 | 0/1 | |
| Site of primary | Glans | Shaft | 0/1 | 0/1 | ||
| Characteristic of primary | Exophytic | Ulcero-infiltrative | 0/1 | 0/1 | ||
| Palpable lymph-nodes | Not palpable | Palpable | 0/1 | 0/1 | ||
| Radiological (USG) | Size of lymph-nodes on USG | <1cm | >1cm | 0/1 | ||
| Fatty hilum | Preserved | Lost | 0/1 | |||
| Radiological (MRI) | Size of lymph-nodes on MRI | <8mm | >8mm | 0/1 | ||
| Irregular outline/necrosis | absent | present | 0/1 | |||
| Pathological | Differentiation | Grade 1 | Grade 2 | Grade 3 | 0/1/2 | 0/1/2 |
| LVI/PNI | Absent | Present | 0/1 | 0/1 | ||
| Total CRiSS score | 0 to 9 | |||||
| CRiSS groups based on scores | ||||||
| LOW RISK GROUP | INTERMEDIATE RISK GROUP | HIGH RISK GROUP | ||||
| 0, 1, 2 | 3, 4 | 5, 6, 7, 8, 9 | ||||
This scoring system has not been validated in any prospective study. We conducted this prospective observational study with an objective of validating the diagnostic accuracy of CRiSS for the prediction of ILNM in squamous cell carcinoma of the penis.
2. Materials and methods
After approval of the study protocol from the institutional review board, we performed a prospective observational study at our institute.
2.1
Sample size calculation
The estimated sample size (n = 100 patients) was determined based on a prevalence rate of 63% for lymph-node metastasis, a per-patient sensitivity of 100%, and a specificity of 72.75% with a confidence interval of ± 0.15 for specificity, as per our previous publication [ ]. This calculation also accounted for an anticipated dropout rate of 5% [ ]. Approximately 35–40 patients with carcinoma penis undergo penectomy with lymph-node dissection at our institute per year. The study was conducted between January 1, 2020, and December 31, 2023, to achieve the desired sample size. All the patients with carcinoma of the penis who presented at our institute during the study period and fulfilled the inclusion criteria were included in the study.
2.2
Inclusion and exclusion criteria
Inclusion criteria were age between 18 and 80 years, biopsy-proven squamous cell carcinoma of the penis, patients who presented primarily to our institute, patients who were not candidates for neoadjuvant chemotherapy, patients who did not opt for options other than surgery, and patients who consented to participation in the study. Exclusion criteria were histology other than squamous cell carcinoma, primary surgery done before presentation to our institute, patients with fixed inguinal nodes or involving skin, patients who presented with recurrence of primary surgery performed before the study period, metastasis on presentation, patients who were not fit for surgery, and patients who did not consent to participation in the study. Written consent for participation, analysis, and publication of the clinical data was obtained from all the patients.
2.3
Study design
On the initial visit, a thorough physical examination was conducted on each patient to record size (cm), site (glans/shaft) and the characteristics (ulcerative/exophytic) of the tumor along with palpation of inguinal lymph-nodes. The size of the lymph-nodes and loss of fatty hilum were noted on ultrasonography. MRI was used as an additional investigation for inguinal lymph-nodes in all cases. MRI was evaluated to address the heterogeneity amongst investigation protocol across various institutions. The size of lymph-nodes and irregular outline or the presence of necrosis was noted in MRI. The criteria of irregular outline/necrosis on MRI were adopted from previously published literature for other cancers [ ]. A wedge biopsy was performed and the type of tumor as well as mild, moderate or poor differentiation along with the presence or absence of LVI/PNI was recorded. Institutional pathologist reviewed the slides of external biopsy report. CRiSS score (0–9) was calculated and group (low/intermediate/high risk) was assigned to all patients.
After a well informed and written consent, all included patients were subjected to primary surgery (partial/total penectomy) and bilateral radical inguinal lymph-node dissection. Partial or total penectomy was performed depending on the feasibility. The radical inguinal lymphadenectomy was performed as described by Daseler et al [ ]. The final histopathology report was recorded and correlated with CRiSS score and groups. Pathological T stage, N stage and the number of dissected lymph-nodes were noted. USG was done by the same team of 2 radiologists throughout the study period. Pathological analysis was done by the same team of experienced onco-pathologists. The radiologists and pathologists were both blinded to the clinical criteria. During the study period, the surgery was conducted by the same group of uro oncologists.
2.4
Statistical analysis
Descriptive analytics was used to quantify and describe the relationships between the selected variables and ILNM. Dichotomous variables were compared by Pearson’s Chi-square test with Yates continuity correction, and Fischer’s exact test was used if at least 1 cell of the table had an expected count smaller than 5. The association between the CRiSS score or groups and ILNM was tested through a logistic regression analysis (HR, 95% CI). The positive predictive value (PPV), negative predictive value (NPV), sensitivity, and specificity of the CRiSS score and groups were calculated. A 2 × 2 table is required for the calculation of sensitivity and specificity, but in cases where there are 3 test results, the result of the equivocal group can be omitted or it can be clubbed with lower or higher group for calculation [ ]. For the calculation of the sensitivity and specificity of each score, we combined the numbers of the rest of the scores to create a 2 × 2 table. The incidence of lymph-node metastasis was 55% (equivocal) in the intermediate CRiSS group; hence, they were grouped with high risk group for calculation of sensitivity and with low risk group for calculation of specificity. A 1-way ANOVA test was used to analyze the difference between the mean number of lymph-nodes dissected among the CRiSS scores and groups [ ]. A p-value of < 0.05 was considered statistically significant for the entire study. The statistical analysis was performed using the SPSS Statistics software for Windows (Version 25.0. Armonk, NY: IBM Corp.)
3
Results
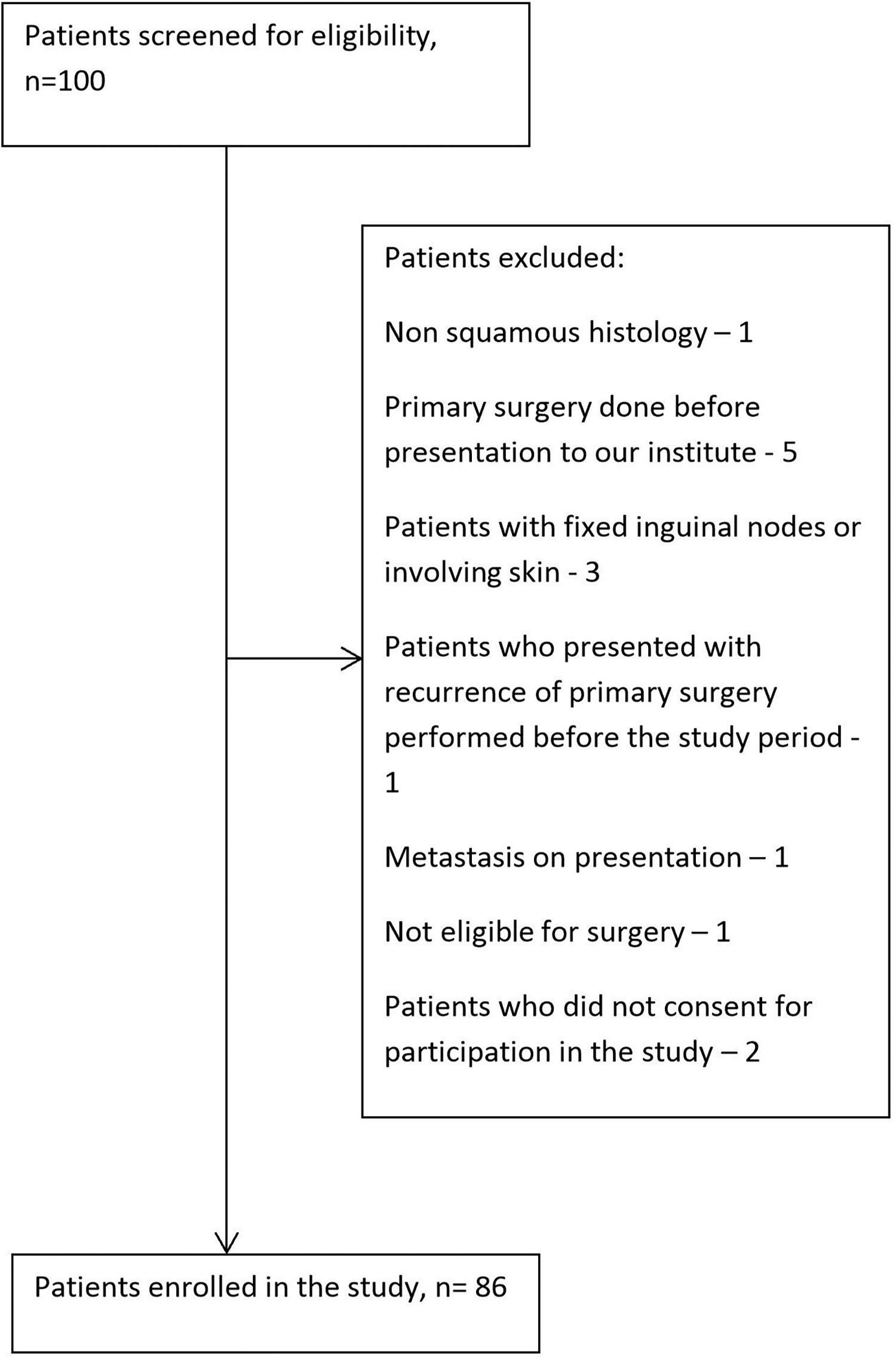
A total of 100 cases of carcinoma penis were enrolled during the study period. Out of them, 86 were deemed eligible for inclusion in the study based on inclusion and exclusion criteria, as shown in Figure 1 .
3.1
Patient characteristics
The characteristics of all the patients are presented in Table 2 . The median age of the patients was 57.45 years (40–75). Tumor larger than 3 cm was present in 38.4% (33 of 86 cases), of which 23 had ILNM. Seventy percent (29 of 41) of the patients had ILNM when they presented with ulcers. ILNM were predominantly found when the tumor was located in the shaft of the penis (73.3%). Inguinal lymph-nodes were palpable in 44 out of 86 (51.2%) patients. ILNM was present in 14 of 42 (33.3%) cases with non-palpable lymph-nodes and in 30 out of 44 patients (68.2%) with palpable lymph-nodes. Lymph-node size of more than 1cm on USG showed an ILNM rate of 70.5%, whereas it was 73.8% if there was a loss of fatty hilum. Ninety-one percent (11 out of 12) of patients with poorly differentiated primary tumors had ILNM, whereas 64.9% (24 out of 37) of patients with moderately differentiated tumors had ILNM. The presence of LVI or PNI could predict ILNM in 32 out of 38 (84.2%) patients. ILNM was found in 41.2%, 45.5%, 55.2%, and 85.7% of patients with T1, T2, T3, and T4 stages, respectively.
| Parameters | Criteria | Total (n) | INLM absent | ILNM present | p-value |
|---|---|---|---|---|---|
| Size of tumor | <3cm | 53 | 32 | 21 | 0.006 |
| >3cm | 33 | 10 | 23 | ||
| Characteristic of growth | Exophytic | 45 | 30 | 15 | 0.001 |
| Ulcero-infiltrative | 41 | 12 | 29 | ||
| Extent of growth | Glans | 41 | 30 | 11 | 0.001 |
| Shaft | 45 | 12 | 33 | ||
| Inguinal lymph node | Not palpable | 42 | 28 | 14 | 0.001 |
| Palpable | 44 | 14 | 30 | ||
| Size of lymph nodes on USG | <1cm | 42 | 29 | 13 | 0.001 |
| >1cm | 44 | 13 | 31 | ||
| Fatty hilum on USG | Preserved | 44 | 31 | 13 | 0.001 |
| Lost | 42 | 11 | 31 | ||
| Size of lymph nodes on MRI | <8mm | 41 | 28 | 13 | 0.001 |
| >8mm | 45 | 14 | 31 | ||
| Characteristics of lymph nodes on MRI | No Irregular outline/necrosis | 42 | 29 | 13 | 0.001 |
| Irregular outline/necrosis | 44 | 13 | 31 | ||
| Grade | 1 | 37 | 28 | 9 | 0.001 |
| 2 | 37 | 13 | 24 | ||
| 3 | 12 | 1 | 11 | ||
| LVI/PNI | Absent | 48 | 36 | 12 | 0.001 |
| present | 38 | 6 | 32 | ||
| T-stage | T1a | 13 | 13 | 0 | 0.001 |
| T1b | 4 | 1 | 3 | ||
| T2 | 33 | 22 | 11 | ||
| T3 | 29 | 10 | 19 | ||
| T4 | 7 | 1 | 6 | ||
| Age | Mean (Years) | 57.45 | 59.75 | 55.90 | 0.373 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree