Highlights
- •
Largest single tertiary center RARC series with the longest follow-up in the U.S.
- •
Extravesical disease and lymph node positivity affect OS and RFS long-term.
- •
Infection and urinary diversion complications are most common for RARC.
- •
Consequences of urinary diversion drive complication rate of RARC.
Abstract
Introduction
To report the long-term outcomes of robot-assisted radical cystectomy (RARC) for the treatment of muscle invasive and high-risk non-muscle invasive bladder cancer.
Methods
We reviewed a single tertiary center database of RARC from 2004 to 2020. Concomitant extended pelvic lymph node dissection and extracorporeal urinary diversion were performed. Cox regression analysis and the Kaplan-Meier method were used to identify factors associated with and report time-to-event estimations of recurrence-free survival and overall survival. Clavien-Dindo complications were identified, categorized, and substratified by time from surgery within 90-days and between 90-days and >5-years postoperatively.
Results
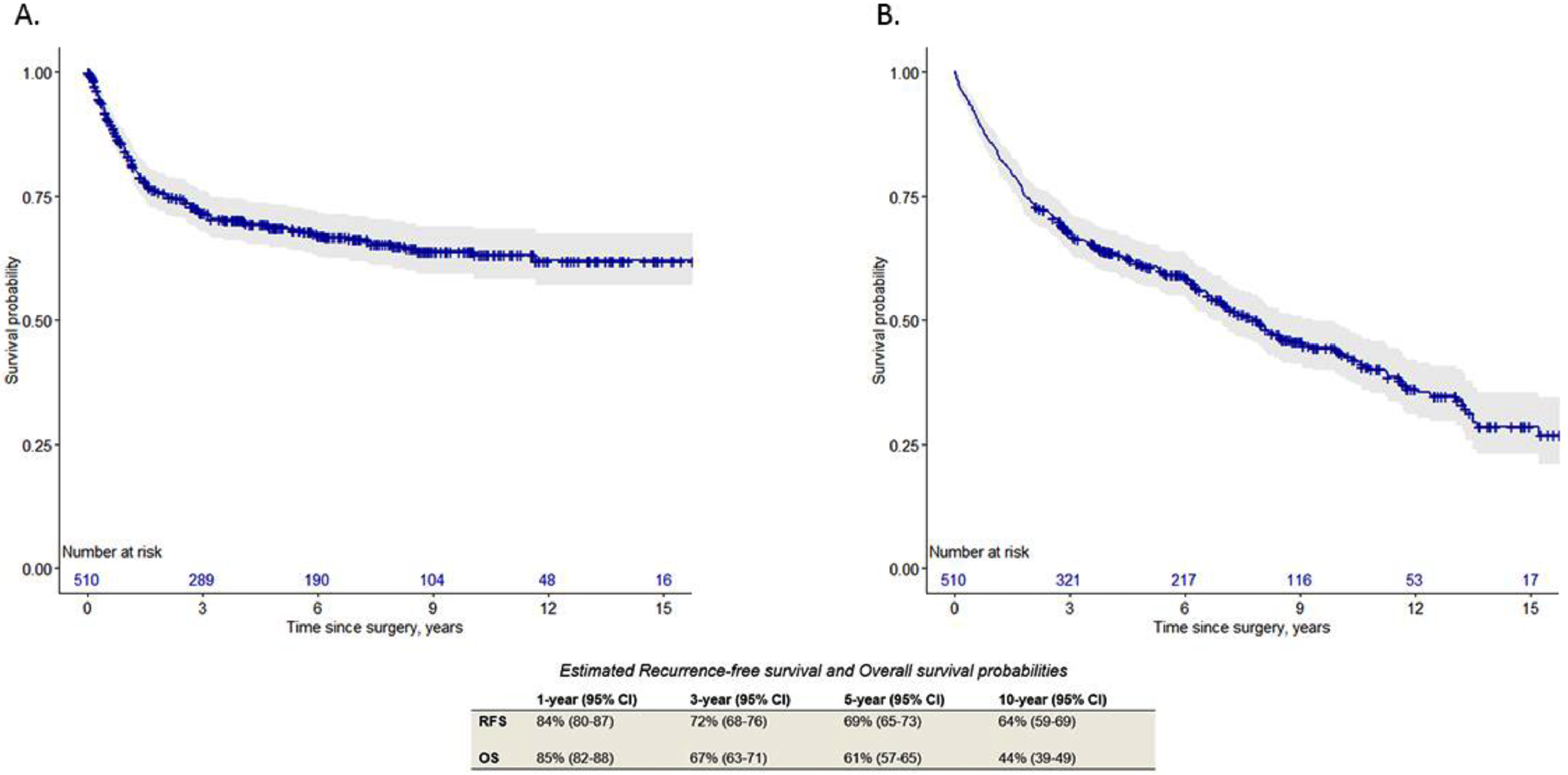
A total of 510 patients with median follow-up of 57.1 months (IQR 21.8–103.6) were included. Continent diversion was performed in 259 (51%) patients. Of the 340 (67%) ≥cT2 patients, 153 (45%) received cisplatin-based neoadjuvant chemotherapy. Recurrence was identified in 157 (31%) patients, and 118 (23%) died from bladder cancer. The overall complication rate was 52% with 267 (41%) major grade ≥ III events. Infectious (25%) and genitourinary (22%) complications were the most common irrespective of the time interval beyond 90-days. The risk of recurrence or death were increased by extravesical disease (HR 1.91 and 1.97, respectively) and lymph node positivity (HR 4.58 and 2.42, respectively) in multivariable analysis (all, P < 0.001). The estimated 5-, and 10-year recurrence-free and overall survival rates were 69% and 64% and 61% and 44%, respectively.
Conclusions
RARC is a durable treatment that optimizes the probability of cure for patients requiring extirpation for bladder cancer. Targeting the modifiable complications of radical surgery may further improve the risk/benefit ratio of RARC.
1
Introduction
For the past 2 decades, radical cystectomy for muscle invasive (MIBC) and high-risk nonmuscle invasive bladder cancer (NMIBC) has shifted towards minimally invasive surgery [ ]. Menon et al. [ ] were the first to demonstrate the feasibility of robot-assisted radical cystectomy (RARC) in 2003. RARC, both retrospectively and in prospective randomized clinical trials, has since demonstrated equivalent oncologic outcomes, a significant reduction in estimated blood loss, and improved convalescence compared to open radical cystectomy (ORC) [ ]. Furthermore, despite an estimated overall complication rate of up to 60%, RARC remains similar in terms of morbidity and may outperform ORC when assessing select complications [ ]. As required of any standard of care treatment, a rigorous evaluation of the long-term efficacy, via comprehensive analysis of patient- and cancer-specific outcomes, is ultimately necessary.
As a high-volume referral center dedicated to the diagnosis, treatment, and management of all urologic malignancies, our operative technique, dedicated urinary diversion team, and postoperative management reflect the modern treatment armamentarium for MIBC and refractory NMIBC. Given more than 80,000 new cases of bladder cancer are diagnosed annually, with up to 40% of patients presenting with or progressing to cT2 disease [ ], RARC involves both life-altering and potentially life-saving treatment. Thus, the objectives of the present study were to report disease-specific indices affecting cancer recurrence, competing and noncompeting risks of mortality, and the landscape of technical and system-based complications in a cohort of patients with extended follow-up who underwent RARC.
2
Methods
2.1
Study design
We reviewed a prospective database (IRB #05148) of 562 consecutive patients who underwent RARC and extracorporeal urinary diversion (ECUD) at a single tertiary referral center between February 2004 and December 2020. Procedures converted to open surgery (n=3) and patients with a preoperative diagnosis of pure adenocarcinoma, squamous, sarcomatoid, or neuroendocrine carcinoma (n=49) were excluded. Primary endpoints were recurrence free survival (RFS) and overall survival (OS). The secondary endpoints included the overall and major complication rates.
2.2
Perioperative management
RARC and extended pelvic lymph node dissection (ePLND) were performed by nine fellowship-trained urologic oncologists using a 6-port transperitoneal approach [ ]. Clinical staging was based on cystoscopic findings, transurethral pathology, and preoperative imaging. Patients with ≥cT2 disease were offered cisplatin-based neoadjuvant chemotherapy (NAC) prior to RARC and were considered to have received NAC if ≥1-cycle of cisplatin- or noncisplatin-based therapy was completed. Nerve sparing was performed if intraoperative evidence suggested that cancer control would not be compromised. Ureteral margin(s) and a urethral margin, if neobladder diversion was planned, were sent for frozen section analysis. A single surgeon (KGC) performed more than 95% of ECUDs with the assistance of intravenous indocyanine green to assess ureteral perfusion prior to ureteroenteric anastomosis after 2016. An institutional enhanced recovery after surgery protocol was introduced in 2014 [ ].
2.3
Follow-up
Disease surveillance was guided by the National Comprehensive Cancer Network guidelines. Specifically, follow-up was conducted every 3- to 6-months for the first 2 years, then biannually thereafter. Surveillance imaging occurred every 6-months for the first 2 years, then for years 3, 4, and 5, a renal ultrasound at the half-year mark and cross-sectional imaging annually. Local and distant recurrences were defined as evidence of radiographic or histologic recurrent disease within the confines of the pelvis or otherwise, respectively. The date of death was recorded using the patient’s death certificate.
2.4
Complications
Complications were identified according to the Clavien-Dindo classification system. Major complications were defined as ≥ grade III. Complications were assigned categorically ( Supplementary table 1 ) and stratified based on occurring >90-days or ≤90-days. Genitourinary, renal, infectious, stomal, and gastrointestinal complications that occurred >90-days were further substratified based on time for the duration of follow-up.
2.5
Statistical analysis
Descriptive statistics were used to summarize all variables. For continuous variables, median and interquartile ranges were reported. OS and RFS estimates were calculated using the Kaplan–Meier method. OS was measured from the date of surgery to date of death from any cause. Patients lost to follow-up or still alive as of their last visit/contact date were censored. RFS was measured from the date of surgery until the date of recurrence. Patients who died without documented recurrence, who were lost to follow-up, or were still alive without documented recurrence as of their last visit/contact date were censored. Death unrelated to bladder cancer was considered a competing risk for cancer-specific death and was estimated using the cumulative incidence function.
Cox proportional hazards regression was used to identify factors associated with OS and RFS. The following variables were included in the univariable analyses based on clinical relevance: age-adjusted Charlson Comorbidity Index (CCI), surgical margin, lymph node status, pathologic stage, pathologic downstaging, use of NAC, and grade of complications. Multicollinearity between covariates was examined using variance inflation factor and correlation analyses. Variables significantly associated with survival in univariable analyses were entered into a multivariable model. Estimates were presented as hazard ratios (HR) with 95% confidence intervals (CI) for univariable and multivariable regression models. Statistical significance was set at P < 0.05. Data management and statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and R version 4.3.0 (R Foundation for Statistical Computing).
3
Results
3.1
Clinicopathologic data
A total of 510 patients, 340 (67%) of whom were ≥cT2, were included in the analysis ( Table 1 ). Median follow-up for the entire cohort was 57.1 (IQR 21.8–103.6) months and 88.7 (IQR 54.2–131.1) months for those that remained alive. Of the 259 (51%) patients with continent ECUDs, 82 (32%) and 177 (68%) underwent Indiana pouch and neobladder creations, respectively. Correlation between CCI score and ECUD are shown in Supplementary table 2 . Pathological upstaging occurred in 180 (35%) patients, 158 (31%) had ≥pT3 disease, and 104 (20%) were pN+ ( Table 1 ). The positive surgical margin (PSM) and local recurrence rates were 3.5% and 7.4%, respectively.
| N = 510 | |
|---|---|
| Age, median (IQR), year | 71 (64–78) |
| Follow-up, median (IQR), month | 57.1 (21.8–103.6) |
| Male, No. (%) | 409 (80) |
| Race, No. (%) | |
| Caucasian | 444 (87) |
| Asian | 30 (5.8) |
| African American | 15 (2.9) |
| Other or unknown | 21 (4.1) |
| Age-adjusted CCI, median (IQR) a | 6 (5–8) |
| BMI, median (IQR), kg/m ² | 26.7 (24.1–30.8) |
| ASA, No. (%) | |
| II | 54 (11) |
| III | 326 (64) |
| IV | 130 (25) |
| Operative time, median (IQR) min | 430 (362–484) |
| EBL, median (IQR) mL | 250 (150–400) |
| Length of hospital stay, median (IQR), day | 7 (6–11) |
| Urinary diversion, No. (%) | |
| Ileal conduit | 251 (49) |
| Indiana pouch | 82 (16) |
| Neobladder | 177 (35) |
| Prior pelvic radiation, No. (%) | 5 (1) |
| Clinical stage, No. (%) | |
| cTa | 24 (4.7) |
| cTis | 27 (5.3) |
| cT1 | 119 (23) |
| cT2 | 276 (54) |
| cT3 | 37 (7.2) |
| cT4 | 27 (5.3) |
| ≥cT2 patients given NAC, No. (%) | 164 (48) |
| Cisplatin-based NAC, No. (%) b | 153 (93) |
| Time to RARC for ≥cT2 patients, median (IQR), month | |
| With NAC b | 5.3 (4.5–6.7) |
| Without NAC | 1.8 (1.4–2.7) |
| ypT0, No. (%) | 47 (29) |
| ypTa, ypTIS, or ypT1, No. (%) | 40 (24) |
| Pathologic stage, No. (%) | |
| pT0 | 83 (16) |
| pTa | 28 (5.5) |
| pTis | 69 (14) |
| pT1 | 55 (11) |
| pT2 | 117 (23) |
| pT3 | 103 (20) |
| pT4 | 55 (11) |
| Pathologic down-stage (No. (%) | 192 (38) |
| Pathologic up-stage No. (%) | 180 (35) |
| Positive surgical margin, No. (%) | 18 (3.7) |
| Lymph node yield, median (IQR) | 32 (22–42) |
| Lymph node stage, No. (%) | |
| NX | 8 (1.5) |
| N0 | 398 (78) |
| N1 | 28 (5.5) |
| N2 | 45 (8.8) |
| N3 | 31 (6.1) |
| Lymph node density, No. (%) | |
| Node density 1–10% | 51 (10) |
| Node density >10% | 53 (10) |
a Patient age at surgery was assigned a weight, where a score of 1 was added for every decade over 40-years. The scores from each comorbidity of CCI along with the weighted scores for age were added to calculate the age-adjusted CCI.
b Received ≥1 cycle of cisplatin-based neoadjuvant chemotherapy.
3.2
Neoadjuvant chemotherapy
Of the 340 ≥cT2 patients, 164 (48%) received NAC. Cisplatin-based NAC was administered to 153/164 (93%) patients. Postoperatively, 87 (53%) of the NAC recipients were downstaged (ypT0-T1). Following NAC + RARC, 9/164 (5.5%) and 46/164 (28%) patients had local or distant recurrences, respectively. In the univariable analysis, NAC reduced the risk of death from any cause (HR 0.61 95%CI: 0.46–0.82, P < 0.001), but did not affect the risk of recurrence ( P = 0.40) ( Supplementary table 3 ). Patients who received NAC and were downstaged had a further reduction in the risk of death (HR 0.40 95%CI: 0.25–0.64, P < 0.001) and recurrence (HR 0.35 95%CI: 0.20–0.61, P < 0.001).
3.3
Recurrence
Disease recurrence was observed in 157 (31%) patients. The 5-, and 10-year RFS estimates were 69% and 64%, respectively ( Fig. 1 A). In the multivariable analysis, lymph node positivity (HR 4.59 95%CI: 3.15–6.71, P < 0.001) and ≥pT3 (HR 1.79 95%CI: 1.18–2.69, P = 0.006) remained significant predictors of worse RFS ( Table 2 ).